Research Articles
Surface Atomistic Structure of Perovskite Quantum Dots: Engineering Strategies for Biomedical Applications
This article provides a comprehensive analysis of the surface atomistic structure of perovskite quantum dots (PQDs) and its pivotal role in determining their optoelectronic properties and functional efficacy.
Standard Reference Materials for Surface Analysis Validation: A Guide for Pharmaceutical and Biomedical Research
This article provides a comprehensive guide for researchers and drug development professionals on the critical role of Standard Reference Materials (SRMs) in validating surface analysis methods.
ISO 18115-2 Decoded: A Guide to Scanning Probe Microscopy Terminology for Biomedical Research
This article provides a comprehensive analysis of the ISO 18115-2 standard for scanning probe microscopy (SPM) terminology, tailored for researchers and professionals in biomedical and drug development.
Electron vs. Ion Spectroscopy: A Comparative Guide to Surface Analysis Techniques for Biomedical Research
This article provides a comprehensive comparison of electron and ion spectroscopy techniques for surface analysis, tailored for researchers and professionals in drug development and biomedical sciences.
Beyond the Baseline: A Modern Framework for Evaluating Detection Limits in Surface Analysis
This article provides a comprehensive guide for researchers and drug development professionals on the critical evaluation of detection limits in surface analysis.
Interlaboratory Comparison in Surface Analysis: A Strategic Framework for Ensuring Data Reliability in Research and Drug Development
This article provides a comprehensive overview of interlaboratory comparisons (ILCs) as critical tools for ensuring the accuracy, reliability, and comparability of analytical results in research and drug development.
Accuracy Assessment in Surface Chemical Measurements: Techniques, Challenges, and Applications in Biomedical Research
This article provides a comprehensive guide to accuracy assessment in surface chemical measurements, tailored for researchers and drug development professionals.
A Modern Framework for Surface Spectroscopy Method Validation: Protocols, Applications, and Compliance
This article provides a comprehensive guide to method validation for surface spectroscopy, addressing the critical needs of researchers and development professionals in regulated environments.
XPS vs AES vs SIMS: A Comprehensive Guide to Surface Analysis Techniques for Biomedical Research
This article provides a detailed comparative analysis of three cornerstone surface analysis techniques—X-ray Photoelectron Spectroscopy (XPS), Auger Electron Spectroscopy (AES), and Time-of-Flight Secondary Ion Mass Spectrometry (ToF-SIMS).
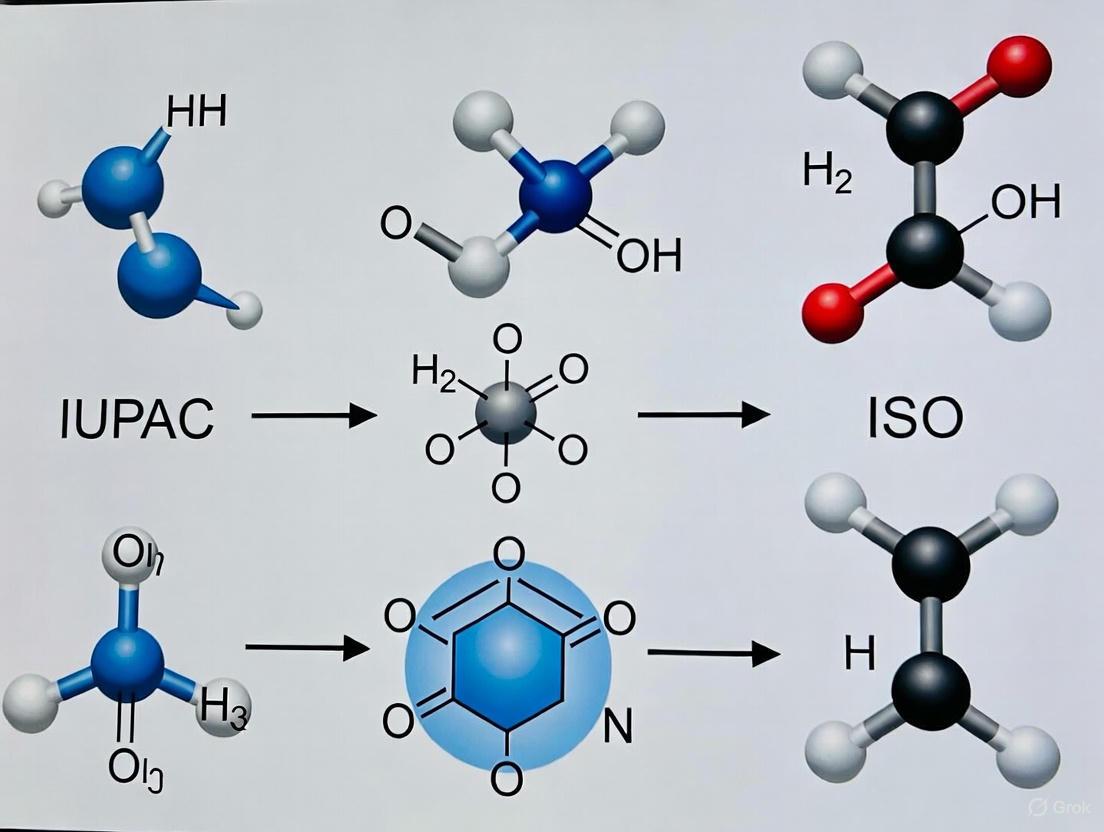
IUPAC vs ISO Terminology for Surface Chemical Analysis: A Practical Guide for Scientists and Regulated Industries
This article provides a comprehensive comparison of IUPAC and ISO terminology standards for surface chemical analysis, tailored for researchers and drug development professionals.