The OA/OAm Ratio in PQD Synthesis: A Foundational Guide for Biomedical Researchers
This article provides a comprehensive analysis of the critical role the oleic acid (OA) to oleylamine (OAm) ratio plays in synthesizing high-performance Perovskite Quantum Dots (PQDs) for biomedical applications.
The OA/OAm Ratio in PQD Synthesis: A Foundational Guide for Biomedical Researchers
Abstract
This article provides a comprehensive analysis of the critical role the oleic acid (OA) to oleylamine (OAm) ratio plays in synthesizing high-performance Perovskite Quantum Dots (PQDs) for biomedical applications. We explore the foundational chemistry of this versatile ligand pair, detailing how their balance dictates PQD characteristics like photoluminescence quantum yield (PLQY), stability, and crystal morphology. The scope extends to practical synthesis methodologies, advanced optimization strategies informed by recent data-driven studies, and a comparative validation of PQD performance against other nanomaterials. Aimed at researchers and scientists in drug development, this review serves as a strategic guide for tailoring PQD properties to meet the stringent demands of biosensing, bioimaging, and clinical diagnostics.
The Chemistry of Control: How OA and OAm Govern PQD Formation and Properties
In the colloidal synthesis of perovskite quantum dots (PQDs), surface ligands are indispensable for modulating material properties and ensuring stability. The ligand pair consisting of oleic acid (OA) and oleylamine (OAm) has emerged as the most prevalent capping agent system used in both research and development [1] [2]. These ligands are integral to the synthesis process, directly influencing the nucleation, growth, and final structural and optical characteristics of the resulting nanocrystals. Their dynamic binding behavior to the perovskite crystal surface dictates critical outcomes, including quantum dot size distribution, crystallographic phase stability, colloidal integrity in solution, and defect formation propensity [1] [3]. A profound understanding of the distinct and synergistic functions of OA and OAm is therefore fundamental for advancing PQD technology, particularly within the context of optimizing their ratio to achieve precise control over the synthesis process and the final product's performance [1].
The Chemistry and Individual Roles of OA and OAm
The OA/OAm pair functions effectively due to the complementary chemical nature of its constituents. Oleic Acid (OA) is a mono-unsaturated fatty acid (C18H34O2) featuring a carboxylic acid head group (-COOH). Oleylamine (OAm), on the other hand, is an unsaturated primary amine (C18H35NH2) with an amine head group (-NH2) [2]. These functional groups are key to their interaction with the precursor ions and the growing perovskite crystal surfaces.
Table 1: Core Functions of the OA/OAm Ligand Pair
| Component | Primary Chemical Function | Role in PQD Synthesis | Impact on PQD Properties |
|---|---|---|---|
| Oleic Acid (OA) | Anionic Capping Agent (X-site binding); Proton donor | Passivates surface lead atoms; Balances charge from OAm+; Contributes to A-site in hybrid PQDs [3]. | Stabilizes crystal structure; Prevents aggregation; Influences photoluminescence quantum yield (PLQY) [4]. |
| Oleylamine (OAm) | Cationic Capping Agent (A-site binding); Weak Reductant; Surfactant | Passivates halide (X-site) anions; Lowers interfacial energy during nucleation; Can reduce metal cations (e.g., Pb2+) [2]. | Controls nucleation & growth kinetics; Determines crystal phase & morphology; Affects particle size & dispersion [1]. |
| OA/OAm Pair | Acid-Base Synergy | Forms ion pairs (oleate and oleylammonium) in situ, creating a complex ligand environment that dynamically binds to the PQD surface [3]. | Governs the dynamic equilibrium of surface coverage; Critical for achieving narrow size distribution and high crystal quality [1] [4]. |
The Impact of the OA/OAm Ratio on PQD Synthesis
The molar ratio of OA to OAm is a critical synthetic parameter, profoundly influencing the reaction pathway and the characteristics of the final PQD product. An imbalance in this ratio can lead to undesirable outcomes such as poor crystallinity, broad size distribution, or rapid degradation [4].
Quantitative Effects and Optimal Ranges
Research indicates that a balanced OA/OAm ratio is crucial for achieving high-quality PQDs. For instance, a deviation from the optimal ratio can significantly reduce the completeness of the cesium precursor conversion from ~98.59% to 70.26%, introducing batch-to-batch inconsistencies and increasing the relative standard deviation of both size distribution and photoluminescence quantum yield (PLQY) to 9.02% and 0.82%, respectively [4]. A proper balance, achieved through novel precursor recipes, has been shown to enable a narrow emission linewidth of 22 nm and a near-unity PLQY of 99% [4].
Consequences of Ratio Imbalance
- Excess Oleic Acid: An over-abundance of OA can lead to under-coordinated lead atoms on the PQD surface due to a lack of amine for effective charge balance. This often results in a high density of trap states, which act as centers for non-radiative recombination, thereby quenching photoluminescence and reducing PLQY [3]. Furthermore, it can destabilize the colloidal solution, prompting aggregation and precipitation.
- Excess Oleylamine: Too much OAm can promote the formation of two-dimensional perovskite phases or other non-cubic structures due to its role as a structure-directing agent [1]. It can also lead to overly rapid reaction kinetics, causing poor size control and a broader particle size distribution. In extreme cases, it can etch the PQDs, degrading their optical properties.
Experimental Protocols: Ligand Engineering in Action
Hot-Injection Synthesis of CsPbX3 QDs
The hot-injection method is a standard technique for producing high-quality, monodisperse PQDs, where ligand engineering plays a central role [1] [2].
Detailed Methodology:
- Preparation of Cs-Oleate Precursor: 0.8 g of Cs2CO3 is mixed with 2.5 mL of OA and 40 mL of OAm in a 100 mL three-neck flask. The mixture is heated to 120°C under inert gas (N2) protection with constant stirring until the Cs2CO3 is completely dissolved, forming a clear solution [1] [2].
- Preparation of PbX2 Precursor: In a separate 50 mL three-neck flask, 0.188 mmol of PbBr2 (or other PbX2 salts) is combined with 5 mL of OAm and 5 mL of OA. This mixture is also heated to 120°C under N2 until the lead salt is fully dissolved.
- Nucleation and Growth: The temperature of the PbX2 precursor solution is rapidly raised to 150–200°C. The Cs-oleate precursor solution (0.4 mL) is then swiftly injected into the reaction flask. Upon injection, the solution immediately turns luminous, indicating the formation of PQDs.
- Termination and Purification: The reaction is quenched after 5–10 seconds by immersing the flask in an ice-water bath. The crude solution is centrifuged at high speed (e.g., 12,000 rpm for 10 minutes) to separate the PQDs from unreacted precursors and excess ligands. The supernatant is discarded, and the pellet is re-dispersed in a non-polar solvent like hexane or toluene for further characterization and application [1].
Ligand Exchange via Alkaline-Augmented Antisolvent Rinsing
A recent advanced protocol demonstrates the critical role of the native OA/OAm ligands and their subsequent exchange for device performance. This method highlights the dynamic nature of the ligand shell and its impact on charge transport [3].
Detailed Methodology:
- PQD Solid Film Assembly: Colloidal PQDs capped with pristine OA and OAm+ ligands are spin-coated onto a substrate to form an "as-cast" solid film.
- Interlayer Rinsing with Alkaline Antisolvent: The solid film is rinsed with a tailored antisolvent, such as methyl benzoate (MeBz), which contains a small, controlled amount of potassium hydroxide (KOH). This alkaline environment facilitates the rapid hydrolysis of the ester antisolvent, generating short-chain conductive ligands (e.g., benzoate). These ligands efficiently substitute the pristine long-chain insulating OA- ligands on the PQD surface [3].
- Mechanism and Outcome: Theoretical calculations reveal that the alkaline environment renders ester hydrolysis thermodynamically spontaneous and lowers the reaction activation energy by approximately nine-fold. This process results in up to twice the conventional amount of conductive ligands capping the PQD surface. The assembled light-absorbing layers exhibit fewer trap-states, homogeneous orientations, and minimal particle agglomerations, which is crucial for high-performance optoelectronic devices like solar cells [3].
The Scientist's Toolkit: Essential Research Reagents
Table 2: Key Reagents for OA/OAm-Based PQD Synthesis
| Reagent / Solution | Technical Function in Synthesis | Rationale & Practical Considerations |
|---|---|---|
| Oleic Acid (OA) | Anionic capping ligand (X-site); Proton source. | High purity (>90%) is critical to prevent side reactions; Must be stored under inert atmosphere to avoid oxidation. |
| Oleylamine (OAm) | Cationic capping ligand (A-site); Surfactant; Weak reductant. | Serves multiple roles; Its purity and freshness directly impact nucleation kinetics and final size distribution. |
| Cesium Carbonate (Cs₂CO₃) | A-site precursor for all-inorganic PQDs. | Forms Cs-oleate in situ with OA; Reactivity is highly dependent on OA/OAm ratio and temperature [4]. |
| Lead Halide Salts (PbX₂) | B-site and X-site precursor (PbBr₂, PbI₂). | The solubility of PbX₂ in the OA/OAm mixture is a key determinant of successful nucleation. |
| Methyl Benzoate (MeBz) | Ester-based antisolvent for ligand exchange. | Used in interlayer rinsing of PQD solid films; Its hydrolysis generates conductive benzoate ligands to replace OA- [3]. |
| Potassium Hydroxide (KOH) | Alkaline additive for antisolvent. | Added to MeBz to create an "Alkali-Augmented Antisolvent Hydrolysis" (AAAH) environment, drastically improving the efficiency of ligand exchange [3]. |
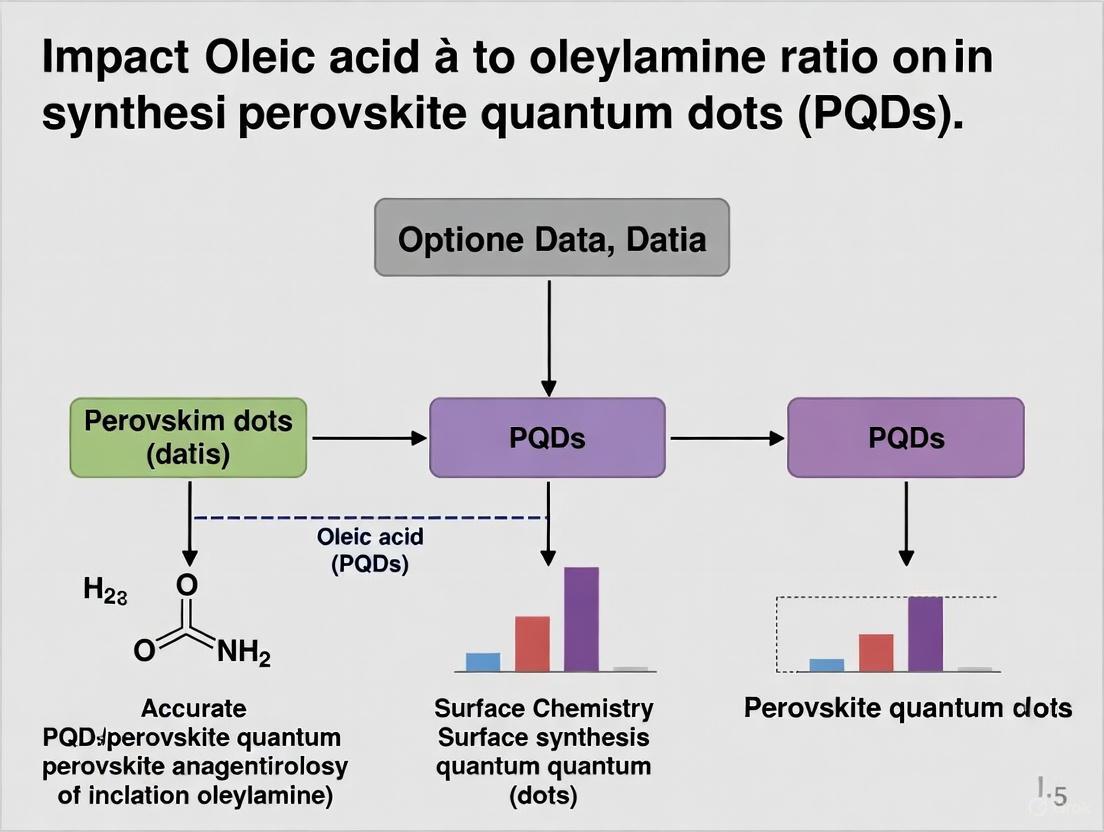
Signaling Pathways and Workflow in Ligand-Assisted Synthesis
The following diagram illustrates the dynamic interactions and decision pathways involved in the OA/OAm ligand-assisted synthesis and post-synthetic treatment of PQDs.
The OA/OAm ligand pair is far more than a simple stabilizer in PQD synthesis; it is a versatile chemical tool that governs reaction thermodynamics, kinetics, and final material properties. The precise ratio of OA to OAm is a fundamental parameter that researchers must optimize to control the nucleation process, crystal growth, surface passivation, and ultimately, the optoelectronic quality of the PQDs. While the native long-chain ligands are excellent for colloidal synthesis, their insulating nature often necessitates post-synthetic exchange to unlock high performance in solid-state devices, a process where the chemistry of the original OA/OAm capping is deeply implicated. Future research exploring more robust and conductive ligand systems, while understanding their dynamic interaction with the classic OA/OAm chemistry, will be crucial for the commercialization of PQD-based technologies.
The synthesis of perovskite quantum dots (PQDs) represents a frontier in nanomaterials research, where precise control over size, shape, and optical properties is paramount for advanced optoelectronic applications. Central to achieving this control is the strategic use of surface ligands that direct nanocrystal growth through facet-specific interactions. The oleic acid (OA) and oleylamine (OAm) ligand pair has emerged as a particularly versatile combination in colloidal synthesis protocols, enabling researchers to manipulate kinetic and thermodynamic parameters throughout the nucleation and growth stages [5].
Within the context of PQD synthesis, the OA/OAm ratio exerts profound influence on the resulting material characteristics, governing everything from quantum confinement effects to phase stability and charge transport properties. This technical guide examines the binding dynamics of this crucial ligand pair, focusing on their facet-specific interactions and the kinetic control they impart during PQD formation. By synthesizing current research findings and experimental approaches, this work provides a comprehensive framework for understanding and manipulating these critical surface interactions to advance PQD research and development.
The OA/OAm Ligand System: Structure and Functions
Oleic acid (cisCH₃(CH₂)₇CHCH)(CH₂)₇COOH) and oleylamine (cisCH₃(CH₂)₇CHCH)(CH₂)₈NH₂) are high-boiling point (≥350°C) ligands that are liquid at room temperature and cost-effective [5]. Despite sharing approximately the same alkyl chain length (~2 nm), they exhibit distinct binding modes, strengths, and additional functionalities that synergistically direct nanoparticle synthesis.
Primary Roles and Binding Modes:
- OA: Functions primarily as a surface capping ligand through carboxylate groups that bind to metal cations on the PQD surface [5] [6]. This binding can occur in various coordination modes (monodentate, bidentate, or bridging), with the specific mode influencing surface stabilization and facet growth rates.
- OAm: Serves dual roles as both a surface ligand through amine group coordination to metal sites and a reducing agent [5]. The amine group can bind to surface atoms, while the alkyl chain provides steric stabilization against aggregation.
- Synergistic Effects: The combination often leads to formation of an acid-base complex (oleylammonium oleate) that may function as a binary capping agent and moderate reductant [5].
Table 1: Primary Functions of OA and OAm in PQD Synthesis
| Function | Oleic Acid (OA) | Oleylamine (OAm) |
|---|---|---|
| Binding Group | Carboxylate (-COO⁻) | Amine (-NH₂) |
| Primary Role | Surface capping, colloidal stability | Surface capping, reducing agent |
| Binding Strength | Moderate to strong, facet-dependent | Moderate, facet-dependent |
| Additional Functions | Precursor complexation | Shape direction, kinetic control |
The different binding strengths and modes of these ligands to specific crystal facets significantly affect reaction kinetics, enabling the production of nanostructures with tailored characteristics [5]. This facet-specific binding is the fundamental mechanism through which the OA/OAm ratio exerts kinetic control over PQD growth and final morphology.
Quantitative Effects of OA/OAm Ratio on PQD Properties
Size and Optical Properties Tuning
The ratio of OA to OAm directly influences PQD size and consequently their optical properties, particularly in methylammonium lead bromide (CH₃NH₃PbBr₃) systems. Research has demonstrated that varying the bad solvent temperature during synthesis while maintaining fixed ligand ratios enables precise size control, but the ligand ratio itself provides an additional dimensional control.
Table 2: Effect of Synthesis Temperature and Implied Ligand Dynamics on CH₃NH₃PbBr₃ PQDs
| Synthesis Temperature (°C) | Average Diameter (nm) | Emission Peak (nm) | Photoluminescence Quantum Yield (%) | Role of OA/OAm |
|---|---|---|---|---|
| 0 | 1.8 | 475 | 74 | Controlled crystallization kinetics |
| 30 | 2.8 | 500 | ~85 | Balanced growth and stabilization |
| 60 | 3.6 | 520 | 93 | Enhanced passivation and defect reduction |
Data adapted from advanced optical studies demonstrates that higher synthesis temperatures yield larger PQDs with red-shifted emission and significantly enhanced quantum yields, achieving up to 93% for 60°C synthesis [7]. This temperature-dependent growth occurs within the kinetic framework established by the OA/OAm ratio, where OAm primarily controls crystallization kinetics while OA suppresses QD aggregation and contributes to colloidal stability [7].
Ligand Ratio Effects on Crystal Phase Stability
The OA/OAm ratio significantly impacts the thermal stability and phase behavior of PQDs, particularly in mixed-cation systems. In situ structural and optical studies of CsₓFA₁₋ₓPbI₃ PQDs across the compositional range reveal distinct thermal degradation mechanisms:
- FA-rich PQDs (with higher OAm binding affinity): Directly decompose into PbI₂ at elevated temperatures (starting ≈150°C) without phase transition [8]
- Cs-rich PQDs: Undergo phase transition from black γ-phase to yellow δ-phase before decomposition [8]
Density functional theory (DFT) calculations confirm that ligand binding strength to FA-rich PQD surfaces is larger than to Cs-rich surfaces, explaining the enhanced thermal stability of FA-rich systems despite their hybrid organic-inorganic composition [8]. This highlights the critical relationship between A-site composition, ligand binding energy, and thermal behavior.
Experimental Protocols for Investigating OA/OAm Binding Dynamics
Synthesis of Size-Tuned CsPbI₃ PQDs
Objective: To prepare monodisperse CsPbI₃ PQDs of different sizes for binary mixing studies [9]
Materials:
- Lead iodide (PbI₂) powder
- Cesium acetate (CsAc) precursor
- 1-octadecene (ODE) solvent
- Oleic acid (OA) and oleylamine (OAm) ligands
Method:
- Dissolve PbI₂ powder in ODE with OA and OAm to form precursor solution
- Degas at 120°C for 1 hour to remove moisture and oxygen
- Inject CsAc precursor predissolved in OA at different temperatures:
- 170°C to produce 14 nm QDs (QD@170)
- 120°C to produce 10 nm QDs (QD@120)
- Perform detailed post-treatment including purification and characterization
Characterization: TEM imaging confirms cubic morphology and size distribution. GIWAXS verifies γ-phase crystal structure. PL spectroscopy shows emission peaks at 688 nm (QD@170) and 662 nm (QD@120) [9]
Binary-Disperse Mixing for Enhanced Packing
Objective: To increase packing density of spin-coated CsPbI₃ PQD films through binary-size mixing [9]
Materials:
- Synthesized QD@170 and QD@120 PQDs
- Non-polar solvent for dispersion
Method:
- Prepare binary mixtures with varying number ratios of QD@170 to QD@120
- Form films by spin-coating precursor solutions (70 mg/mL) at 1000 rpm for 10 seconds, followed by 2000 rpm for 7 seconds
- Characterize packing structure using GISAXS
- Fit scattering data with three-phase model (two monosize phases + one binary mixing phase)
Analysis:
- Maximum packing volume fraction (37.1%) achieved at number ratio 0.64 (14 nm)/0.36 (10 nm)
- Molecular dynamics simulations reveal enhanced face-face contact in binary mixtures
- Densely packed films exhibit suppressed trap-assisted recombination and longer carrier lifetime [9]
Ligand Exchange for Enhanced Water Compatibility
Objective: To replace native OA/OAm ligands with multidentate alternatives for biological applications [6]
Materials:
- CsPbBr₃ PQDs with native OA/OAm ligands
- Succinic acid (SA), folic acid (FA), ethylenediamine tetra-acetic acid (EDTA), glutamic acid (GA)
- N-Hydroxy succinimide (NHS)
- Bovine serum albumin (BSA) protein
Method:
- Synthesize OA-capped CsPbBr₃ PQDs using standard hot-injection method
- Exchange native ligands with dicarboxylic acid ligands (SA, FA, EDTA, GA)
- Characterize binding strength through theoretical calculations and optical measurements
- React SA-treated PQDs with NHS in water to form multidentate ligand passivation
- Test bioconjugation with BSA protein model
Characterization:
- Theoretical calculations show stronger binding between perovskite and SA compared to OA
- SA-treated PQDs show significant improvement in fluorescence and stability
- NHS activation enables covalent bioconjugation with biomolecules [6]
Kinetic Control and Facet-Specific Interactions
The OA/OAm ligand pair exerts kinetic control over PQD synthesis through several interconnected mechanisms that influence both nucleation and growth stages. Understanding these processes is essential for achieving tailored nanostructures with precise morphological control.
Diagram 1: Kinetic Control Pathways in PQD Synthesis. The OA/OAm ratio determines ligand availability for modulating nucleation kinetics and facet-specific growth.
The kinetic pathways illustrated above operate through several specific mechanisms:
5.1 Nucleation Control: OAm primarily controls crystallization kinetics during the nucleation stage [7]. The amine group facilitates precursor solubility and modulates supersaturation rates, which is particularly crucial in reprecipitation methods where precursor solution is injected into a bad solvent [7].
5.2 Growth Direction: The differential binding of OA and OAm to specific crystal facets directs anisotropic growth. OA shows preferential binding to certain crystal planes, while OAm binds more strongly to others [5]. This facet-specific adsorption creates differential growth rates along crystal axes, enabling morphology control.
5.3 Stability and Defect Passivation: Proper OA/OAm balance ensures effective surface passivation during growth, reducing surface defects and enhancing photoluminescence quantum yield [7]. The synergistic interaction between the ligands creates a protective layer that stabilizes the PQD surface against degradation while maintaining colloidal stability.
Research Reagent Solutions Toolkit
Table 3: Essential Materials for Investigating OA/OAm Binding Dynamics in PQD Synthesis
| Reagent/Chemical | Function in Research | Application Context |
|---|---|---|
| Oleic Acid (OA) | Primary capping ligand, surface passivation | Standard component in most PQD synthesis protocols |
| Oleylamine (OAm) | Co-ligand, reducing agent, shape director | Paired with OA for size and morphology control |
| Lead Halides (PbX₂) | Perovskite precursor | Required for APbX₃ structure formation |
| Cesium Carbonate/Cesium Acetate | Cesium source for all-inorganic PQDs | Hot-injection synthesis of CsPbX₃ PQDs |
| Formamidinium Halides | A-site cation source for hybrid PQDs | Synthesis of FAPbX₃ with narrow bandgaps |
| 1-Octadecene (ODE) | High-boiling nonpolar solvent | Reaction medium for high-temperature synthesis |
| Succinic Acid (SA) | Short-chain bidentate ligand | Ligand exchange for enhanced stability [6] |
| N-Hydroxy Succinimide (NHS) | Carboxyl group activator | Bioconjugation after ligand exchange [6] |
| Cellulose Nanocrystals | Long-chain binding ligands | OA/OAm-free stabilization for ultra-stable PQD papers [10] |
The binding dynamics of the oleic acid/oleylamine ligand pair in perovskite quantum dot synthesis represent a sophisticated toolbox for materials scientists seeking precise control over nanomaterial properties. Through facet-specific interactions and kinetic control mechanisms, the OA/OAm ratio influences critical PQD characteristics including size distribution, crystal phase, optical properties, and thermal stability. The experimental methodologies and research reagents detailed in this technical guide provide a foundation for systematic investigation of these ligand dynamics.
As PQD research advances toward commercial applications, understanding and manipulating these surface interactions will grow increasingly important for achieving optimal material performance in solar cells, light-emitting diodes, quantum technologies, and biological applications. The multifaceted role of these common ligands underscores the complexity of nanomaterial synthesis while offering versatile strategies for material optimization through surface engineering approaches.
The synthesis of high-performance metal halide perovskite quantum dots (PQDs) necessitates precise control over reaction kinetics and surface chemistry to achieve superior photoluminescence quantum yield (PLQY) and stability. The oleic acid (OA) and oleylamine (OAm) ligand pair has been identified as a critical component in this process, where their acid-base complex formation fundamentally alters precursor reactivity and dictates final PQD characteristics. This whitepaper delineates how the OA/OAm ratio, a key parameter emerging from data-driven interrogation of the chemical synthesis space, serves as a powerful tool for tailoring PQD properties. By integrating domain knowledge with exploratory data analysis (EDA), researchers can systematically optimize this ligand pair to accelerate the development of advanced optoelectronic devices [11].
Colloidal synthesis of nanostructured materials has revolutionized the production of semiconductors, with PQDs standing out due to their exceptional optoelectronic properties. These zero-dimensional materials exhibit distinct chemical, physical, electrical, and optical characteristics not found in their bulk counterparts, making them highly promising for applications spanning solar cells, light-emitting diodes (LEDs), lasers, and quantum technologies [12]. The controlled synthesis of these nanoparticles in organic solvents relies heavily on the choice of surfactants to tune composition, morphology, and ultimately, performance [13].
The dynamic instabilities at the PQD surface and the complex chemistry involved in their formation have, until recently, hindered their commercial translation [12]. The synergistic use of the OA and OAm ligand pair has emerged as a versatile strategy to address these challenges. This combination, belonging to two different categories of molecules (carboxylic acid and amine), aims not only to control nanoparticle size and shape and prevent aggregation but also to actively modulate the chemical environment of the precursor materials [13]. The resulting acid-base interaction forms a complex that acts as a binary capping agent and potential reductant, whose properties can be finely tuned by adjusting the component ratio, thereby offering a powerful lever to direct synthesis outcomes [11] [13].
Chemical Foundation of the OA-OAm Complex
Formation Mechanism and Binding Modes
The interaction between oleic acid (OA) and oleylamine (OAm) is fundamentally an acid-base reaction. The carboxylic acid group (-COOH) of OA donates a proton to the amine group (-NH₂) of OAm, resulting in the formation of an oleate-oleylammonium ion pair. This reaction can proceed during the initial stages of the synthesis process when both ligands are present in the reaction mixture [13].
The resulting acid-base complex exhibits distinct binding characteristics compared to the individual ligands. This complex can serve as a binary capping agent that binds to specific crystallographic facets of the growing PQD nuclei. The different binding strengths and distinct binding modes of OA and OAm on these specific facets are crucial for controlling the reaction kinetics and directing anisotropic growth, which is essential for producing nanostructures with tailored characteristics [13]. The complex itself may also possess reducing capacity, though this is generally lower than that of pure OAm, which is often noted for its independent reducing function [13].
Table: Functional Roles of the OA/OAm Ligand Pair in PQD Synthesis
| Component | Chemical Function | Impact on PQD Synthesis |
|---|---|---|
| Oleic Acid (OA) | Carboxylic acid ligand; coordinates via carboxylate group | Passivates surface cations; controls growth rate; prevents aggregation |
| Oleylamine (OAm) | Alkylamine ligand; coordinates via amine group | Acts as a reducing agent and surface ligand; influences crystal phase |
| OA-OAm Complex | Acid-base ion pair (oleate-oleylammonium) | Binary capping agent; modulates precursor reactivity; controls morphology |
Impact on Precursor Reactivity and Reaction Kinetics
The formation of the OA-OAm complex directly impacts the chemical nature of the precursors in solution. By binding to metal halide precursors, the complex can alter their solvation energy and reduction potential, effectively tuning their reactivity. This moderated reactivity is essential for achieving a controlled nucleation and growth process, preventing the explosive nucleation that leads to polydisperse PQDs.
The following diagram illustrates the sequence of chemical interactions from the initial ligand pairing to the final PQD product, highlighting the key role of the OA/OAm ratio.
Diagram: The pathway through which the OA/OAm ratio influences final PQD quality. The OA/OAm ratio is the critical parameter governing this process. A specific ratio determines the equilibrium concentration of the free ligands and the acid-base complex, which in turn dictates the surface binding dynamics and the final nanoparticle properties [11]. An imbalance in this ratio can lead to insufficient surface passivation, resulting in low PLQY, or overly rapid growth, yielding large, non-emissive particles.
Quantitative Effects of the OA/OAm Ratio on PQD Properties
The optimization of the OA/OAm ratio is not a trivial task, as it influences multiple, often interdependent, synthesis outcomes. Exploratory Data Analysis (EDA) has been employed to systematically interrogate this complex chemical space, moving beyond traditional trial-and-error approaches [11]. This data-driven methodology evaluates feature correlations and uses regression models to identify critical synthesis parameters, with the oleic acid/oleylamine ligand pair consistently emerging as a dominant factor [11].
The permutation importance analysis from such studies quantitatively confirms that the OA/OAm ratio is a top-tier predictor for key performance metrics like Photoluminescence Quantum Yield (PLQY). Subsequent refinement of this ratio through a multi-stage optimization sequence has been shown to significantly enhance PLQY, leading to the discovery of ideal parameter values with minimal experimental resources [11].
Table: Impact of OA/OAm Ratio on Synthesis Outcomes and PQD Properties
| OA/OAm Ratio | Impact on Reaction Kinetics | Resulting PQD Morphology | Typical PLQY Outcome |
|---|---|---|---|
| High OA | Slower growth, reduced nucleation burst | Smaller, isotropic dots | Moderate, can be unstable |
| High OAm | Faster reaction, increased reduction potential | Anisotropic, larger particles | Variable, often lower |
| Balanced/Optimized | Controlled nucleation & balanced growth | Uniform, monodisperse QDs | High (Significantly enhanced) [11] |
The efficacy of combining categorical and continuous features in the synthesis model underscores the value of domain expertise in data preprocessing and feature selection [11]. The ideal ratio appears to facilitate an optimal surface coverage where the different binding modes of the two ligands work in concert to effectively passivate the PQD surface, minimizing surface defects that non-radiatively recombine charge carriers.
Experimental Protocols for OA/OAm Ratio Optimization
EDA-Guided Synthesis Workflow
This protocol outlines a data-driven method for optimizing the OA/OAm ratio to maximize PLQY in PQDs, as validated in recent literature [11].
Targeted Dataset Assembly:
- Compile a dataset from existing literature and controlled experiments. Key features should include categorical variables (e.g., solvent type, metal precursor) and continuous variables (e.g., OA/OAm ratio, reaction temperature, precursor concentration). The target output variable is PLQY.
Exploratory Data Analysis (EDA) and Feature Correlation:
- Employ statistical software (e.g., Python with Pandas, Scikit-learn) to evaluate correlations between all synthesis parameters and the PLQY.
- Use permutation importance analysis within a regression model (e.g., Random Forest) to identify and rank the most critical parameters influencing PLQY. This step quantitatively confirms the OA/OAm ratio as a key factor.
Three-Stage Ratio Refinement:
- Stage 1 (Coarse Screening): Perform a broad experimental screen across a wide range of OA/OAm ratios (e.g., from 1:10 to 10:1) while keeping other parameters constant.
- Stage 2 (Focused Optimization): Narrow the range around the most promising ratios identified in Stage 1 and conduct replicates to establish statistical significance.
- Stage 3 (Validation): Validate the optimal ratio in a full synthesis procedure, confirming its impact on both PLQY and stability.
The workflow for this data-driven optimization is summarized in the following diagram.
Diagram: The EDA-guided workflow for optimizing the OA/OAm ratio.
Standard Hot-Injection Synthesis with Parameterized Ligands
This is a generalized protocol for PQD synthesis where the OA/OAm ratio can be systematically varied.
- Research Reagent Solutions & Essential Materials:
Table: Key Reagents for PQD Synthesis via Hot-Injection
Reagent/Material Function in Synthesis Example Metal Halide Salt Provides metal cation (e.g., Pb²⁺, Cs⁺) for perovskite structure Lead bromide (PbBr₂), Cesium carbonate (Cs₂CO₃) Organic Ammonium Salt Provides organic cation for hybrid perovskites Oleylammonium bromide (OAmBr) Oleic Acid (OA) Ligand; surface passivation; controls growth kinetics Typically 90% technical grade Oleylamine (OAm) Ligand; reducing agent; surface passivation Typically 90% technical grade High-Boiling Solvent Reaction medium Octadecene (ODE)
Precursor Solution Preparation:
- Cesium Oleate Precursor: Load Cs₂CO₃ into a flask with OA and ODE. Heat under inert gas until dissolved.
- Lead Halide Precursor: Load PbBr₂ into a separate flask with dried ODE. Degas under vacuum. Add precise, variable volumes of OA and OAm according to your experimental design, followed by OAmBr. Heat under inert gas until the salt is completely dissolved.
Reaction and Purification:
- Stabilize the lead halide precursor solution at the desired reaction temperature (e.g., 150-180 °C).
- Rapidly inject the cesium oleate precursor solution into the vigorously stirred lead halide solution.
- Let the reaction proceed for 5-30 seconds before cooling the reaction flask in an ice-water bath.
- Purify the resulting PQDs by centrifugation, decanting the supernatant, and re-dispersing the pellet in an anhydrous solvent.
The formation of the OA-OAm acid-base complex is a fundamental chemical process that exerts profound influence on precursor reactivity and the subsequent trajectory of PQD synthesis. The ratio of OA to OAm is not merely a compositional variable but a powerful, versatile tool that governs reaction kinetics, morphological development, and ultimate optoelectronic performance. The integration of data-driven strategies, such as EDA, with deep domain knowledge provides a robust pathway for elucidating complex synthesis parameter spaces. This synergistic approach, centered on understanding and optimizing the OA/OAm ligand pair, is pivotal for overcoming current instabilities and accelerating the commercialization of high-performance PQDs in next-generation optoelectronic devices.
The synthesis of perovskite quantum dots (PQDs) with superior optoelectronic properties is critically dependent on the precise control of surface chemistry, particularly the ratio of organic ligands used as capping agents. Oleic acid (OA) and oleylamine (OAm) constitute the most prevalent ligand pair for colloidal PQD synthesis, dynamically coordinating to crystal surfaces to modulate growth, passivate defects, and influence colloidal stability. This whitepaper delineates the profound impact of the OA/OAm stoichiometry on pivotal PQD characteristics, including photoluminescence quantum yield (PLQY), crystal size distribution (CSD), and structural crystallinity. Drawing upon recent experimental and data-driven studies, we establish that the ligand ratio is not a mere supplementary parameter but a fundamental determinant that governs nucleation kinetics, defect formation, and ultimate device performance. The insights herein are framed within the broader context of optimizing perovskite research for next-generation optoelectronic applications.
The emergence of metal halide perovskite quantum dots (PQDs) has revolutionized optoelectronics, offering exceptional properties such as high photoluminescence quantum yields (PLQYs), narrow emission linewidths, and widely tunable bandgaps [12] [14]. The functional prowess of these nanocrystals, however, is intimately tied to the synthetic protocols employed in their creation. Among the various synthetic parameters, the engineering of surface ligands stands out as an indispensable strategy for boosting the luminescence performance and environmental stability of PQDs [14]. Ligands are molecules that attach to the PQD surface, forming a coordination complex that is crucial for directing nucleation and growth, passivating surface defects, and ultimately determining the crystal's size, morphology, and stability [14].
The ligand pair of oleic acid (OA) and oleylamine (OAm) has become the conventional standard in both hot-injection and ligand-assisted reprecipitation (LARP) synthesis methods [14] [11]. In this dynamic system, OA (a carboxylic acid) typically chelates with surface lead atoms, while OAm (a primary amine) interacts with halide ions via hydrogen bonding [14]. The balance between these two interactions, governed by the OA/OAm ratio, is a critical but complex factor. It directly influences the precursor reactivity, the kinetics of crystal growth, and the final surface chemistry of the PQDs. An imbalance can lead to poor surface passivation, facilitating non-radiative recombination pathways, or result in uncontrolled growth and aggregation, broadening the crystal size distribution and degrading performance [15] [14] [11]. This whitepaper synthesizes current research to provide a definitive guide on correlating the OA/OAm ratio to the key properties of PLQY, size distribution, and crystallinity.
The Ligand Ratio as a Synthetic Control Knob
Fundamental Interactions and Mechanisms
The OA and OAm ligands engage in a dynamic equilibrium at the growing crystal surface. The ratio of these ligands determines the net charge and the steric hindrance around the PQD, which in turn controls the rate at which new monomers are added to the crystal lattice. Furthermore, the ligand ratio can influence the formation energy of surface defects. A non-optimal ratio may leave under-coordinated lead atoms or halide vacancies unpassivated, which act as traps for charge carriers and dramatically reduce PLQY [14] [16]. Exploratory data analysis (EDA) of PQD synthesis has pinpointed the OA/OAm ligand pair as a key factor, with its ratio being a critical parameter requiring refinement to enhance PLQY [11].
The diagram below illustrates the core mechanism of how the OA/OAm ratio governs the synthesis outcome and final PQD properties.
Quantitative Impact of Ligand Ratio on Key PQD Properties
The following tables consolidate quantitative findings from recent studies on how the OA/OAm ratio modulates critical PQD properties.
Table 1: Impact of OA/OAm Ratio on MAPbBr3 PQD Synthesis using Short-Chain Amines [15]
| Ligand System | Amine/Acid Ratio | Average Size | PLQY | Size Distribution | Key Finding |
|---|---|---|---|---|---|
| Oleylamine (OAm) / Oleic Acid (OA) | Not fully optimized | Broad distribution, large particles | Lower than OcAm | Wide, with co-formation of large particles | Conventional long-chain OAm leads to poorer size control. |
| Octylamine (OcAm) / Oleic Acid (OA) | Optimized (~5.9:1 molar ratio) | ~7.8 nm | High (>80%) | Monodisperse, no large particles | Short-chain OcAm yields higher quality, more uniform PQDs. |
Table 2: Ligand Engineering Strategies and Correlated PQD Properties [14] [16] [17]
| Ligand Strategy | Targeted Property | Mechanism of Action | Effect on PLQY & Stability |
|---|---|---|---|
| Lewis Base Passivation (e.g., Trioctylphosphine - TOP) | PLQY, Defect Passivation | TOP coordinates with undercoordinated Pb²⁺ sites, reducing surface traps and halide loss. | Near-unity PLQY (97.9%) reported for blue-emitting CsPbBr₃ QDs; enhanced color stability in LEDs [16]. |
| Inorganic Capping (e.g., PbSO₄-oleate, SiO₂) | Stability, Halide Exchange | Forms a physical barrier that impedes ion migration and protects against environmental factors [18] [17]. | Increased activation energy for halide exchange; improved moisture and air stability [18] [17]. |
| Short-Chain Ligand Exchange | Charge Transport, Packing Density | Replaces long-chain OA/OAm to reduce interparticle distance, enhancing tunneling and film conductivity [9]. | Improved power conversion efficiency in solar cells (PCE of 14.42%) due to suppressed trap-assisted recombination [9]. |
Experimental Protocols: Optimizing and Analyzing the Ligand Ratio
Low-Temperature Synthesis of Monodisperse MAPbBr3 PQDs
This protocol, adapted from Wang et al., highlights the optimization of the amine-to-acid ratio for improved size uniformity [15].
- Chemicals: Lead bromide (PbBr₂, ≥99.999%), methylamine bromide (MABr, ≥99.95%), oleylamine (OAm, 90%), oleic acid (OA, 90%), octylamine (OcAm, ≥99.0%), N,N-Dimethylformamide (DMF, ≥99.8%), Toluene (≥99.8%).
- Synthesis Procedure:
- Precursor Preparation: Co-dissolve 0.044 mmol PbBr₂, 0.056 mmol MABr, and the desired molar amounts of amine (OAm or OcAm) and OA in 10 mL DMF using ultrasonic agitation to form a clear, transparent solution. systematically vary the
C_Amine/C_OAmolar ratio (e.g., from 2:1 to 10:1) and the totalC_Ligand/C_Precursorratio. - Antisolvent Crystallization: Pre-heat 5 mL of toluene to a set temperature (e.g., 48 °C) with vigorous stirring. Quickly inject 0.25 mL of the precursor solution into the toluene.
- Growth and Purification: Allow the reaction to proceed for 10 minutes. Then, centrifuge the solution at 14,000 rpm for 10 minutes. Discard the supernatant and re-disperse the pellet in toluene for characterization.
- Precursor Preparation: Co-dissolve 0.044 mmol PbBr₂, 0.056 mmol MABr, and the desired molar amounts of amine (OAm or OcAm) and OA in 10 mL DMF using ultrasonic agitation to form a clear, transparent solution. systematically vary the
- Key Analysis: Use UV-Vis absorption and photoluminescence (PL) spectroscopy to monitor the band edge and emission profile. Transmission electron microscopy (TEM) is critical for assessing the size distribution and monodispersity.
Data-Driven Optimization of the OA/OAm Ratio
A modern EDA-guided approach can efficiently navigate the complex synthesis parameter space [11].
- Workflow:
- Dataset Assembly: Construct a targeted dataset from historical synthesis experiments, featuring parameters like OA/OAm ratio, reaction temperature, precursor concentration, and outcomes like PLQY and FWHM.
- Feature Correlation: Employ regression models and permutation importance analysis to identify the OA/OAm ratio as a critically important feature.
- Sequential Refinement: Execute a three-stage experimental sequence focusing specifically on refining the OA/OAm ratio, while other parameters are held constant.
- Validation: Confirm the model predictions with targeted experiments to discover ideal ligand ratio values that lead to enhanced PLQY.
Post-Synthetic Ligand Exchange for Enhanced Charge Transport
This protocol is essential for device applications where efficient charge transport between PQDs is paramount [9].
- Chemicals: CsPbI₃ PQDs (synthesized via standard hot-injection), Methyl acetate (MeOAc, 99.5%), Short-chain ligand (e.g., Formamidinium Iodide or Guanidinium Iodide).
- Procedure:
- Film Formation: Spin-coat a layer of pristine OA/OAm-capped CsPbI₃ PQDs onto a substrate.
- Ligand Treatment: Drop-cast a solution of the short-chain ligand dissolved in MeOAc onto the freshly deposited PQD film. Let it sit for 20-30 seconds to allow ligand exchange.
- Rinsing: Spin the film to remove the excess solution and rinse gently with pure MeOAc to wash away the displaced long-chain ligands and by-products.
- Repetition: Repeat the layer-by-layer deposition and ligand exchange process to build a thick, densely packed film.
- Verification: Grazing-incidence small-angle X-ray scattering (GISAXS) can quantify the increased packing density and reduced interparticle distance [9].
The Scientist's Toolkit: Essential Research Reagents
Table 3: Key Reagents for Ligand Ratio Studies in PQD Research
| Reagent / Material | Function / Role in Research | Key Consideration |
|---|---|---|
| Oleylamine (OAm) | Long-chain amine ligand; binds to halide ions, controls crystal growth, affects surface charge. | Technical grade (≈70-90%) often used; ratio with OA is critical for morphology [15] [14]. |
| Oleic Acid (OA) | Long-chain carboxylic acid ligand; chelates Pb²⁺ ions, stabilizes precursors and growing nanocrystals. | Must be used with OAm for balanced charge; excess can lead to etching [14] [11]. |
| Short-Chain Amines (e.g., Octylamine) | Alternative amine ligand; can yield higher quality, monodisperse PQDs with smaller sizes [15]. | Shorter carbon chain improves size control and may enhance charge transport but can affect colloidal stability. |
| Lewis Base Ligands (e.g., Trioctylphosphine) | Post-synthetic passivator; coordinates with Pb²⁺ to fill halide vacancies, dramatically boosting PLQY [16]. | Effective for defect passivation in ultra-small QDs, crucial for achieving high-efficiency blue emission. |
| Lead Halides (PbX₂) | Pb²⁺ precursor for the B-site in ABX₃ perovskite structure. | High purity (≥99.998%) is required to minimize unintended impurities and defects. |
| Cesium Carbonate (Cs₂CO₃) | Cs⁺ precursor for the A-site in all-inorganic CsPbX₃ PQDs. | Reacts with OA to form Cs-oleate, which is injected into the lead halide precursor solution. |
| Alkyl Ammonium Halides | Short-chain ligands for post-synthetic exchange; replace OAm to improve inter-dot coupling [9]. | Reducing ligand chain length decreases interparticle distance, fostering better charge transport in films. |
The correlation between the oleic acid/oleylamine ligand ratio and the fundamental properties of perovskite quantum dots is unequivocal and profound. This whitepaper has established that meticulous tuning of this ratio is a prerequisite for achieving high PLQY, narrow crystal size distribution, and optimal crystallinity. The move towards short-chain and multidentate ligands, as well as data-driven optimization, represents the forefront of PQD research, enabling enhanced performance and stability in optoelectronic devices. Future research will likely focus on developing a more fundamental, predictive understanding of ligand-surface interactions and exploiting machine learning to navigate the vast multi-parameter synthesis space. Ultimately, mastering ligand engineering is the key to unlocking the full commercial potential of perovskite quantum dots in lighting, displays, and energy generation.
The development of high-performance optoelectronic devices based on perovskite quantum dots (PQDs) hinges on resolving a fundamental contradiction: the very chemical agents that ensure colloidal stability during synthesis simultaneously undermine the electronic performance of the final material. This trade-off represents one of the most significant challenges in advancing PQD technologies from laboratory demonstrations to commercial applications. At the heart of this conflict lies the standard ligand system of oleic acid (OA) and oleylamine (OAm), which serves as a cornerstone in colloidal synthesis methodologies. These long-chain organic ligands expertly passivate surface defects and prevent agglomeration during the nucleation and growth phases, enabling the production of stable, high-purity PQD inks with exceptional optical properties [19] [20]. However, their insulating nature creates a significant electronic barrier in solid-state films, hampering charge transport and extraction—properties essential for devices such as solar cells, photodetectors, and light-emitting diodes [20].
The OA/OAm ratio during synthesis directly influences this balance, acting as a critical parameter that determines both the morphological quality of the resulting nanocrystals and their ultimate device performance. This technical guide examines the mechanistic basis of this trade-off, presents quantitative data on its operational impacts, and outlines advanced material strategies that are pushing the boundaries of what is achievable with PQD-based optoelectronics. By framing this discussion within the context of the OA/OAm ligand system, we provide researchers with a fundamental understanding of how synthetic choices propagate through to device-level performance, enabling more rational design of next-generation PQD materials.
The Dual Role of Surface Ligands in PQD Systems
Surface ligands in perovskite quantum dot systems perform two essential but often contradictory functions, creating an intrinsic tension between material processability and electronic performance.
Ensuring Colloidal Stability: The Synthetic Necessity
During synthesis and processing, ligands serve as steric barriers that prevent uncontrolled growth and agglomeration of nanocrystals. The dynamic binding nature of OA and OAm to the PQD surface allows for reversible adsorption and desorption, facilitating proper crystal growth while maintaining nanoscale dimensions [19]. This ligand shell is crucial for achieving narrow size distributions (typically 5-10% relative standard deviation) and high photoluminescence quantum yields (PLQY) often exceeding 80-90% in solution [19]. The carboxylic acid group of OA and the amine group of OAm coordinate with surface atoms, effectively passivating undercoordinated sites that would otherwise act as non-radiative recombination centers [20]. This surface passivation is particularly important for metal-halide perovskites, which, despite their defect-tolerant nature, still require careful surface management to achieve optimal optical properties.
Compromising Optoelectronic Performance: The Electronic Consequence
When PQDs are assembled into solid-state films for device applications, the same ligand shell that ensured colloidal stability becomes a significant liability. The long hydrocarbon chains of OA and OAm (typically 18 carbon atoms) create substantial inter-dot spacing of 1-2 nanometers, which dramatically reduces wavefunction overlap between adjacent dots [20]. This physical separation leads to exceptionally low charge carrier mobility (often 10-4-10-2 cm²/V·s) due to inefficient hopping transport between quantum-confined states [20]. Furthermore, the insulating nature of these aliphatic chains creates a high energy barrier for charge injection and extraction at electrode interfaces. The problem is compounded by the fact that ligand shells are often imperfect, with dynamic binding leading to variable surface coverage and the emergence of trap states during film processing [19]. Even with complete coverage, the dipole moments of the ligand molecules can create energetic disorder at interfaces, further complicating charge transport processes.
Table 1: Impact of Standard Ligands on Key PQD Properties
| Property | Role in Colloidal Stability | Impact on Optoelectronic Performance |
|---|---|---|
| Inter-dot Distance | Prevents aggregation (<2nm spacing) | Inhibits charge transport (>1nm barrier) |
| Surface Passivation | Redces surface defects (high PLQY) | Creates insulating barrier |
| Film Morphology | Enables uniform deposition | Limits dense packing (voids/cracks) |
| Energy Alignment | Minimal effect | Introduces interface dipoles |
Quantitative Analysis of the OA/OAm Ratio Impact
The ratio of oleic acid to oleylamine during synthesis serves as a critical control parameter that directly influences PQD formation, stability, and final device performance. Systematic investigations have revealed how this balance affects multiple aspects of material properties.
Synthesis Outcomes and Ligand Removal Efficiency
Recent studies on FAPbI₃ PQD synthesis via the ligand-assisted reprecipitation (LARP) method have quantified the relationship between initial ligand ratios and outcomes after purification treatments. Using methyl acetate (MeOAc) as a purification solvent, researchers have demonstrated that approximately 85% of initial ligands can be removed through liquid/solid purification processes [20]. However, the initial OA/OAm ratio significantly influences the efficiency of this process and the quality of the resulting films.
Table 2: Ligand Removal Efficiency and Film Properties with Varying MeOAc Purification
| MeOAc Volume (mL) | Ligand Removal Efficiency | Film Quality Observations | Inter-dot Spacing |
|---|---|---|---|
| 1 (LP1) | ~70% | Poor morphology, cracking | Minimal reduction |
| 3 (LP3) | ~85% | Improved density, fewer cracks | Significantly reduced |
| 5 (LP5) | ~90% | Over-purification, increased defects | Minimal ligands |
The purification process itself introduces secondary effects—while removing insulating ligands, it can create undercoordinated surface atoms that act as charge traps unless properly repassivated with shorter ligands [20]. This highlights the nuanced balance required in ligand management: insufficient removal limits conductivity, while excessive removal creates electronic defects that promote non-radiative recombination.
Device Performance Metrics
The ultimate validation of ligand engineering strategies comes from completed devices, where the impact of OA/OAm ratios and subsequent ligand exchange processes becomes quantitatively apparent in performance metrics. Photovoltaic devices incorporating FAPbI₃ PQDs with optimized multiligand exchange (using 3-mercaptopropionic acid and formamidinium iodide) have demonstrated ≈28% improvement in power conversion efficiency compared to devices with conventional OA/OAm ligands [20]. This enhancement stems primarily from a 2 mA cm⁻² increase in current density, directly attributable to improved charge transport through the reduced inter-dot spacing [20].
Beyond efficiency gains, proper ligand management significantly improves operational stability—devices with optimized short ligands demonstrate reduced hysteresis in current-voltage characteristics and maintain performance over extended operational periods [20]. Electrochemical impedance spectroscopy reveals that hybrid MPA/FAI passivation improves thin-film conductivity and quality by reducing inter-dot spacing and defects, thereby mitigating vacancy-assisted ion migration [20].
Advanced Strategies for Overcoming the Trade-off
Ligand Exchange and Engineering Approaches
The limitations of standard OA/OAm systems have driven the development of advanced ligand strategies that maintain sufficient passivation while enabling enhanced electronic communication between PQDs.
Solid-State Ligand Exchange: This approach involves first synthesizing PQDs using conventional OA/OAm ligands to achieve optimal size and morphological control, then performing a post-deposition treatment to replace these long-chain ligands with more compact alternatives [21] [20]. The sequential solid-state multiligand exchange process for FAPbI₃ PQDs utilizes solutions of 3-mercaptopropionic acid (MPA) and formamidinium iodide (FAI) in methyl acetate to systematically replace octylamine (OctAm) and oleic acid (OA) [20]. This strategy has demonstrated particular success in photovoltaic applications, where it enables improved charge transport while maintaining sufficient surface passivation.
Bidentate Ligand Systems: Ligands featuring multiple binding groups (such as dithiols or dicarboxylates) offer enhanced binding affinity compared to monodentate OA/OAm systems [21]. This stronger coordination reduces ligand desorption during processing, leading to more stable surface passivation. Ethanedithiol (EDT) has been successfully employed in electrohydrodynamic printing of NCs, enabling conductive patterns without high-temperature annealing [21].
Hybrid Organic-Inorganic Passivation: Combining short organic ligands with inorganic salts (e.g., lead iodide, cesium iodide) creates a mixed passivation layer that addresses both organic and inorganic surface sites [20]. This approach has demonstrated remarkable success in photovoltaic devices, where FAI/MPA co-passivation reduces interfacial recombination while facilitating charge transport [20].
Material and Architectural Innovations
Beyond ligand chemistry, material compositions and device architectures offer additional pathways for mitigating the stability-performance trade-off.
Lead-Free Perovskite Derivatives: Materials such as Cs₃Bi₂X₉ and CsSnX₃ offer inherently higher stability in aqueous environments, reducing the stringent passivation requirements of lead-based perovskites [19]. While these systems typically exhibit lower photoluminescence quantum yields (50-70% compared to 80-90% for lead-based PQDs), their enhanced stability enables thinner ligand shells and improved charge transport [19].
Matrix Encapsulation Strategies: Embedding PQDs within stable matrices such as metal-organic frameworks (MOFs) or inorganic oxides provides environmental protection while allowing electronic interaction [19]. The PQD@MOF composites in particular have demonstrated exceptional selectivity in complex matrices for sensing applications, surpassing carbon quantum dots and traditional semiconductor QDs in both sensitivity and versatility [19].
Ratiometric Sensing Designs: For detection applications, advanced device architectures that incorporate internal reference signals can compensate for PQD instability in complex matrices [19]. These designs mitigate the impact of variable ligand coverage on signal output, enhancing reliability for applications such as heavy metal ion detection in industrial wastewater [19].
Ligand Engineering Strategy Diagram
Experimental Protocols: Methodologies for Balanced PQD Development
Sequential Multiligand Exchange for FAPbI₃ PQDs
This protocol outlines the synthesis and sequential ligand exchange process for formamidinium lead iodide (FAPbI₃) PQDs, which has demonstrated significant improvements in photovoltaic device performance [20].
Materials Requirement:
- Lead(II) iodide (PbI₂, 99.9% trace metals basis)
- Formamidinium iodide (FAI, CH₅IN₂, 99.9%)
- Oleic acid (OA, C₁₈H₃₄O₂, 97%)
- Octylamine (OctAm, C₈H₁₉N, 99%)
- 3-Mercaptopropionic acid (MPA, C₃O₂H₆S, 90%)
- Solvents: acetonitrile (ACN), toluene, hexane, chloroform, methyl acetate (MeOAc)
Synthesis Procedure:
- Precursor Preparation: Dissolve PbI₂ (0.1 mmol, 0.045 g) in ACN (2 mL) with OA (200 μL) and OctAm (20 μL) under stirring.
- FAI Solution Preparation: Separately mix FAI (0.0137 g, 0.08 mmol) with OA (40 μL), OctAm (6 μL), and ACN (0.5 mL).
- Reaction: Add the FAI solution dropwise to the PbI₂ solution with continuous stirring.
- Precipitation: Inject the resulting mixture into preheated toluene (10 mL, 70°C) under rapid stirring, followed by immediate quenching in an ice/water bath.
- Purification: Collect the precipitate via ultracentrifugation at 9000 rpm for 15 minutes. Redisperse in hexane (1 mL) and centrifuge again at 6000 rpm for 10 minutes to remove agglomerated particles.
Ligand Exchange Protocol:
- Liquid Purification: Add varying volumes of MeOAc (1-5 mL) to the colloidal solution before centrifugation to remove excess ligands.
- Solid-State Exchange: Treat spin-coated PQD films with a solution of MPA and FAI in MeOAc for 30-120 seconds.
- Rinsing: Remove excess ligand solution by spinning with appropriate solvents (methyl acetate for MPA/FAI).
- Layer-by-Layer Processing: Repeat deposition and exchange cycles to build thick, compact films.
Characterization Methods:
- HRTEM: Determine size distribution and interplanar spacing (∼11 nm average size for FAPbI₃ PQDs).
- 1H NMR: Quantify ligand removal efficiency (∼85% achieved with MeOAc).
- Photoluminescence Spectroscopy: Assess optical properties and defect states.
- Electrochemical Impedance Spectroscopy: Measure thin-film conductivity and charge transport properties.
Ligand-Exchange-Assisted Electrohydrodynamic Printing
This advanced printing methodology enables direct patterning of functional PQD structures with sub-micron resolution, combining the precision of electrohydrodynamic printing with the performance benefits of in situ ligand exchange [21].
Materials System:
- Colloidal nanocrystal inks (Ag, Au, PbS, ZnO NCs) with OA/OLAM ligands
- Compact ligand reagents: NH₄SCN, ethanedithiol (EDT), tetrabutylammonium iodide (TBAI), PbI₂
- Non-polar solvents: dodecane, octane
Fabrication Process:
- Ink Preparation: Prepare stable dispersions of OA-capped NCs in non-polar solvents at optimized concentrations for EHDP.
- Electrohydrodynamic Printing: Utilize pulsed voltages to eject NC droplets from meniscus at nozzle tip, achieving feature sizes down to 70 nm.
- In Situ Ligand Exchange: Immediately after printing, treat structures with compact ligand solutions (30-120 seconds).
- Rinsing and Drying: Remove excess ligands and byproducts with appropriate solvents.
- Multi-layer Integration: Repeat process with different NC inks and ligands to construct complex device architectures.
Performance Outcomes:
- Achieved electrical conductivity of (1.99\times {10}^{7}\,{{{\rm{S}}}}/{{{\rm{m}}}}) for Ag NC films with NH₄SCN treatment.
- Demonstrated all-printed PbS NC/ZnO NC IR photodiodes at sub-10-µm pixel sizes.
- Enabled operation at lower dark currents and faster photoresponse than printed photoconductors.
Ligand-Exchange-Assisted Nano-Printing Workflow
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 3: Key Research Reagents for PQD Ligand Engineering
| Reagent/Material | Chemical Function | Role in Addressing Trade-off | Application Context |
|---|---|---|---|
| Oleic Acid (OA) | Carboxylic acid surfactant | Primary capping ligand for synthesis | Colloidal stability during growth |
| Oleylamine (OAm) | Amine surfactant | Co-ligand for surface passivation | Size and morphology control |
| 3-Mercaptopropionic Acid (MPA) | Short-chain thiol ligand | Charge transport enhancement | Solid-state ligand exchange |
| Formamidinium Iodide (FAI) | Organic halide salt | Surface passivation and repair | Defect reduction in films |
| Methyl Acetate (MeOAc) | Polar solvent | Selective ligand removal | Purification and exchange processes |
| Ethanedithiol (EDT) | Bidentate thiol ligand | Strong-binding compact ligand | Conductivity enhancement |
| NH₄SCN | Inorganic salt | Metal coordination ligand | Electrical conductivity in metal NCs |
| Tetrabutylammonium Iodide (TBAI) | Halide salt | Iodide source for passivation | Surface defect healing |
The fundamental trade-off between colloidal stability and optoelectronic performance in perovskite quantum dots continues to drive innovative research at the intersection of materials chemistry, surface science, and device engineering. While the OA/OAm ligand system remains foundational for synthesizing high-quality PQDs, emerging strategies that decouple the stabilization function from charge transport limitations show significant promise. The sequential multiligand exchange approach demonstrates that properly engineered hybrid passivation can simultaneously address both requirements, enabling ≈28% efficiency improvements in photovoltaic devices [20].
Future research directions should focus on developing dynamic ligand systems that provide stabilization during synthesis but can be thermally or photochemically modified after film formation to enhance conductivity. Additionally, computational screening methods for identifying ideal ligand combinations could accelerate the discovery of novel materials that optimize this balance. As lead-free perovskite derivatives mature, their inherently different surface chemistry may offer alternative pathways for resolving this fundamental challenge [19] [22].
The integration of in situ characterization techniques during ligand exchange processes will provide deeper mechanistic understanding of surface transformations, enabling more precise control over the final material properties. As these advanced strategies mature, the path toward PQD-based optoelectronics that do not force a compromise between processability and performance becomes increasingly clear, paving the way for next-generation quantum dot devices across energy, sensing, and display applications.
Synthesis in Practice: Optimizing OA/OAm Ratios Across Key PQD Fabrication Techniques
The hot-injection synthesis of perovskite quantum dots (PQDs) has established itself as a foundational method for producing nanocrystals with exceptional optoelectronic properties. Central to this process is the strategic use of organic ligands, particularly the dynamic pair of oleic acid (OA) and oleylamine (OAm), which govern nucleation, growth, and final nanocrystal characteristics. Within the broader thesis investigating the impact of the OA:OAm ratio on PQD synthesis, this technical guide elucidates the precise protocols and mechanistic principles that enable researchers to harness this ratio for synthesizing high-purity, monodisperse nanocrystals. The ligand ratio is not merely a supplementary parameter but a primary determinant of surface energy, defect passivation, and morphological control, directly influencing the charge carrier dynamics and structural integrity of the resulting PQDs [23] [24]. This guide provides a comprehensive framework for mastering ligand engineering in hot-injection synthesis, from fundamental binding thermodynamics to advanced protocols for CsPbBr3 and hybrid organic-inorganic PQDs.
Theoretical Foundation: Ligand Binding and Surface Dynamics
The Chemistry of OA and OAm on PQD Surfaces
In the ionic crystal lattice of lead halide perovskites (CsPbX3, MAPbX3), surface sites consist of under-coordinated Pb²⁺ cations and halide anions (X⁻). OA and OAm interact with these surfaces through distinct but complementary mechanisms:
- Carboxylic Acid Binding (OA): Oleic acid typically binds as a carboxylate (oleate) to under-coordinated Pb²⁺ sites, acting as an L-type ligand through electron pair donation [23] [24].
- Amine Binding (OAm): Oleylamine often binds as an alkylammonium cation (oleylammonium) to halide-deficient surfaces, forming an ion pair with surface halides [25]. Studies quantifying ligand binding thermodynamics on CsPbBr3 QDs reveal that oleylamine exchanges exergonically with incoming amine ligands (K_eq = 2.52 for undec-10-en-1-amine), indicating favorable binding [25].
The ratio of OA to OAm determines the protonation state of the ligand shell and the electrostatic environment around the nanocrystal. An imbalance can lead to incomplete surface coverage, increased defect density, and poor colloidal stability [23] [24].
Impact of Ligand Ratio on Nucleation and Growth
The classical LaMer model describes nanocrystal formation as a process of initial supersaturation, rapid nucleation, and subsequent growth. Ligands profoundly influence each stage:
- Nucleation Phase: Ligands coordinate with precursors in solution, modulating monomer reactivity and the critical concentration required for nucleation [26]. A higher OAm ratio typically accelerates nucleation by enhancing precursor solubility.
- Growth Phase: The ligand ratio determines surface energy and growth kinetics. An optimal ratio ensures a balanced passivation of both cationic (Pb²⁺) and anionic (Br⁻) surface sites, facilitating steady, diffusion-controlled growth and suppressing Ostwald ripening [15] [26].
- Final Morphology: The binding affinity and steric bulk of the ligand shell dictate the nanocrystal's final shape and size distribution. Strongly bound ligands favor isotropic growth, leading to cubic morphology, while unbalanced ratios can cause anisotropic growth or aggregation [27] [26].
Table 1: Thermodynamic Parameters of Ligand Exchange on CsPbBr3 QDs at 25°C
| Incoming Ligand | Native Ligand | Equilibrium Constant (K_eq) | Binding Nature |
|---|---|---|---|
| 10-Undecenoic Acid | Oleate | 1.97 ± 0.10 | Exergonic, Reversible |
| Undec-10-en-1-amine | Oleylamine | 2.52 | Exergonic, Reversible |
| 10-Undecenylphosphonic Acid | Oleate | Irreversible | Strong, Irreversible |
The following diagram illustrates the mechanistic role of ligands during the hot-injection synthesis process, from precursor coordination to final surface passivation.
Experimental Protocols: Optimized Synthesis via Ligand Engineering
Protocol 1: CsPbBr3 QDs with Tunable Amine Ligands
This protocol, adapted from Ji et al. (2023), demonstrates how different amine ligands—oleylamine (OAm), octylamine (OcAm), and phenylethylamine (PEA)—influence the optical properties and morphology of CsPbBr3 QDs [27].
Synthesis of Cs-Oleate Precursor:
- Dissolve 0.36 g Cs₂CO₃ (0.1 M) in 15 mL 1-octadecene (ODE) and 1.5 mL OA in a 100 mL three-neck flask.
- Degas for 10 minutes at 120°C under Ar flow until complete dissolution.
QD Synthesis via Hot-Injection:
- Load 0.188 mmol PbBr₂, 15 mL ODE, 1.5 mL OA, and 1.5 mL of the chosen amine ligand (OAm, OcAm, or PEA) into a separate 100 mL three-neck flask.
- Degas at 120°C for 30 minutes under Ar flow.
- Raise the temperature to 170°C and swiftly inject 1.5 mL of the pre-prepared Cs-oleate solution.
- React for 10 seconds before cooling in an ice-water bath.
Purification:
- Centrifuge the crude solution at 10,000 rpm for 5 minutes.
- Re-disperse the precipitate in 5 mL hexane and centrifuge again at 6,000 rpm for 3 minutes.
- Collect the supernatant containing the purified CsPbBr3 QDs.
Key Findings:
- Solution PLQY: QDs with long-chain OAm and OcAm ligands showed superior photoluminescence quantum yield (PLQY) in solution (~95% and ~93%, respectively) compared to PEA-capped QDs (~85%).
- Film PLQY: In solid films, PEA-capped QDs exhibited the highest PLQY (~80%) due to better packing and reduced inter-dot distance, while OAm and OcAm films dropped to ~70% and ~75%, respectively [27].
Protocol 2: Monodisperse MAPbBr3 PQDs Using Short-Chain Amines
This protocol, from Wang et al. (2021), highlights the advantages of using short-chain octylamine (OcAm) over long-chain oleylamine (OAm) for synthesizing methylammonium lead bromide (MAPbBr3) PQDs at lower temperatures (48°C) [15].
Precursor Solution Preparation:
- Co-dissolve 0.044 mmol PbBr₂, 0.056 mmol MABr, 0.094 mmol OcAm, and 0.016 mmol OA in 10 mL DMF using ultrasonic agitation until a clear, transparent solution forms.
Crystallization and Purification:
- Quickly inject 0.25 mL of the DMF precursor solution into 5 mL of toluene preheated to 48°C under vigorous stirring.
- Maintain reaction for 10 minutes.
- Centrifuge the solution at 14,000 rpm for 10 minutes to discard large aggregates.
- Collect the supernatant containing monodisperse MAPbBr3 PQDs.
Optimized Parameters:
- Molar Ratio of OcAm to OA (COcAm/COA): Optimal at 5.9.
- Molar Ratio of Total Ligands to Pb²⁺ Precursor (CL/CP): Optimal at 2.5.
- Reaction Temperature: 48°C.
Performance Outcomes:
- The OcAm-capped MAPbBr3 PQDs achieved a high PLQY of 87% and exhibited superior stability under moisture and laser irradiation compared to OAm-capped counterparts [15].
Table 2: Comparative Analysis of Hot-Injection Synthesis Protocols
| Parameter | Protocol 1: CsPbBr₃ [27] | Protocol 2: MAPbBr₃ [15] |
|---|---|---|
| Target PQD | CsPbBr₃ | MAPbBr₃ |
| Precursor Solvent | 1-Octadecene (ODE) | N,N-Dimethylformamide (DMF) |
| Reaction Temperature | 170°C | 48°C |
| Key Ligand Ratio Finding | Long-chain amines (OAm, OcAm) superior in solution; short-chain (PEA) superior in films | Optimal COcAm/COA = 5.9; CL/CP = 2.5 |
| Achieved PLQY | Up to 95% (Solution), ~80% (Film) | 87% |
| Key Advantage | Ligand-dependent application (solution vs. film devices) | High monodispersity without large particles; enhanced stability |
The Scientist's Toolkit: Essential Research Reagents
Successful hot-injection synthesis relies on high-purity reagents and precise material functions. The following table catalogs the essential components for ligand-engineered PQD synthesis.
Table 3: Essential Reagents for Ligand-Engineered Hot-Injection Synthesis
| Reagent / Material | Function & Role in Synthesis | Exemplary Purity & Handling |
|---|---|---|
| Cesium Carbonate (Cs₂CO₃) | Cs⁺ precursor for all-inorganic PQDs [27]. | 99.99%, stored in a desiccator; dissolved in OA/ODE for Cs-oleate [27]. |
| Lead Bromide (PbBr₂) | Pb²⁺ and halide (Br⁻) source [27] [15]. | ≥99.99%, anhydrous; often used as the limiting reagent [27]. |
| Methylammonium Bromide (MABr) | Organic A-site cation source for hybrid PQDs [15]. | ≥99.99%; must be handled in an inert, dry atmosphere to prevent degradation [15]. |
| Oleic Acid (OA) | Primary X-type ligand; passivates Pb²⁺ sites, controls growth, imparts colloidal stability [24] [23]. | Technical grade (90-98%); must be purified or tested for peroxide formation. |
| Oleylamine (OAm) | L-type ligand/alkylammonium cation; passivates halide sites, enhances precursor solubility [23] [25]. | Technical grade (70-90%); degassed before use to remove water and impurities. |
| 1-Octadecene (ODE) | High-boiling, non-coordinating solvent for hot-injection [27] [28]. | ≥90%; must be purified and degassed to remove peroxides and polar impurities. |
| Octylamine (OcAm) | Short-chain amine alternative; can yield smaller, more monodisperse PQDs with higher PLQY [15]. | ≥99%; used in optimized molar ratios with OA [15]. |
The precise engineering of the OA:OAm ratio in hot-injection synthesis transcends routine optimization, emerging as a critical strategy for controlling the fundamental properties of perovskite quantum dots. As detailed in this guide, the ligand ratio directly dictates nucleation kinetics, growth thermodynamics, surface passivation efficacy, and ultimately, the optoelectronic performance and application potential of PQDs. The protocols and data presented provide a roadmap for synthesizing high-purity nanocrystals tailored for specific applications, from solution-processed light-emitting diodes to densely packed photovoltaic films. Future research directions will likely focus on developing even more robust ligand systems, such as multidentate and zwitterionic ligands, to further enhance environmental and operational stability [12] [24]. Mastering ligand engineering is, therefore, not merely a synthetic step but a cornerstone for advancing PQD technology toward commercial viability and integration into next-generation optoelectronic devices.
Tuning the OA/OAm Balance in Ligand-Assisted Reprecipitation (LARP) for Room-Temperature Synthesis
The synthesis of metal halide perovskite nanocrystals (PNCs) represents a rapidly advancing frontier in nanomaterials research, driven by their exceptional optoelectronic properties, including high photoluminescence quantum yield (PLQY), narrow emission bandwidth, and easily tunable bandgaps [29] [30]. Among the various synthesis techniques, ligand-assisted reprecipitation (LARP) has emerged as a particularly accessible and scalable method, performed at room temperature without the need for inert atmospheres or complex apparatus [31]. The LARP method hinges on the precise coordination of surface ligands that control crystallization, stabilize the resulting nanocrystals, and determine their final morphological and optical characteristics [29] [32]. Central to this process is the binary ligand system comprising oleic acid (OA) and oleylamine (OAm), whose balance critically influences every stage of nanocrystal formation, from precursor dissolution to final nanocrystal stability and phase [32]. This technical guide examines the sophisticated role of the OA/OAm equilibrium within the LARP protocol, providing researchers with a detailed framework for optimizing this critical parameter to synthesize high-quality PNCs for advanced applications.
Chemical Fundamentals of OA and OAm in LARP
Individual Roles and Synergistic Effects
In the LARP process, oleic acid (OA) and oleylamine (OAm) perform distinct yet interconnected chemical functions. OA, a carboxylic acid, primarily acts as a steric stabilizer, preventing nanocrystal coagulation through repulsive forces generated by its long alkyl chain [29] [32]. OAm, a primary amine, plays an integral role in controlling crystallization and directing nanocrystal growth [29] [32]. The synergistic effect of this ligand pair arises from their acid-base equilibrium, which creates a dynamic environment at the nanocrystal surface crucial for achieving monodisperse, stable colloids [32].
The binding mechanisms of these ligands to the perovskite surface are multifaceted. According to the Covalent Bond Classification, OA typically functions as an X-type ligand, forming a covalent bond with surface sites, while OAm acts as an L-type ligand, donating an electron pair to uncoordinated surface metal orbitals [29]. The surface of CsPbX3 NCs can be conceptualized as [CsPbX3][PbX2]{AX}, where A represents oleylammonium ions and X represents halides and/or oleate ions, highlighting the direct incorporation of these ligands into the surface chemistry [32].
Precursor Solubilization and Reaction Kinetics
A fundamental requirement in LARP is achieving sufficient precursor solubility in polar solvents such as dimethylformamide (DMF) or dimethyl sulfoxide (DMSO). The OA/OAm binary system enhances precursor dissolution approximately 50-fold compared to solvent alone [32]. Specifically, OAm significantly improves PbX2 solubility through complex formation, while both ligands facilitate cesium salt incorporation into the reaction mixture. This enhanced solubility directly impacts reaction kinetics by promoting a more homogeneous precursor distribution, enabling controlled supersaturation upon injection into the anti-solvent—a critical factor in achieving uniform nucleation and growth [32].
Quantitative Effects of OA/OAm Ratio on PNC Properties
The balance between OA and OAm concentrations exerts profound influence on the structural and optical properties of resulting PNCs. Even slight deviations from optimal ratios can trigger phase transitions, morphological changes, and significant variations in photoluminescence efficiency.
Table 1: Effects of Varying OA/OAm Ratios on CsPbBr3 Nanocrystal Properties
| OA/OAm Ratio | Nanocrystal Phase | Morphology | PLQY | Stability | Key Observations |
|---|---|---|---|---|---|
| OAm-rich | Cs4PbBr6 (non-perovskite) | Various shapes, often non-uniform | Low (< 20%) | Low | Lead-depleted phase formation; Poor optical properties [32] |
| Balanced | CsPbBr3 (perovskite) | Uniform nanocubes | High (> 80%) | High | Optimal passivation; Narrow size distribution [32] |
| OA-rich | CsPbBr3 (perovskite) | Nanocubes, sometimes aggregated | Moderate to High | Moderate | Improved steric hindrance but potential packing issues [32] |
The data reveals that OAm-rich conditions particularly risk transforming the luminescent CsPbBr3 perovskite phase into the non-luminescent Cs4PbBr6 phase [32]. This transition occurs because excess OAm extracts PbBr2 from the crystal lattice, disrupting the perovskite structure. Conversely, a properly balanced OA/OAm ratio ensures appropriate surface passivation of both cationic (Pb²⁺) and anionic (Br⁻) sites, yielding CsPbBr3 nanocubes with high PLQY and superior colloidal stability [32].
Table 2: Impact of Absolute Ligand Concentrations on NC Characteristics
| Concentration Level | Size Control | Size Distribution | Reaction Yield | Practical Implications |
|---|---|---|---|---|
| Low | Poor control | Broad | Low | Insufficient surface coverage leads to aggregation [32] |
| Optimal | Good control | Narrow (PDI < 5%) | High | Balanced nucleation and growth rates [31] [32] |
| Excessive | Constrained growth | Narrow but small sizes | Variable | May limit growth or induce secondary phases [32] |
Experimental Protocols for OA/OAm Optimization
Standardized LARP Procedure with Parameterized Ligand Ratios
Materials Required:
- Lead bromide (PbBr₂, ≥99.9%)
- Cs₂CO₃ (≥99.9%)
- Oleic acid (OA, technical grade 90%)
- Oleylamine (OAm, technical grade 70%)
- Dimethylformamide (DMF, anhydrous)
- Toluene (anhydrous)
- Methyl acetate (MeOAc, for purification)
Synthesis Procedure:
- Precursor Solution Preparation: Dissolve PbBr₂ (0.2 mmol, 0.0734 g) in 2 mL DMF with constant stirring.
- Ligand Addition: Add OA and OAm in predetermined molar ratios (see Table 3) to the PbBr₂ solution. The total ligand volume typically ranges between 100-500 μL to maintain precursor solubility while avoiding excessive ligand loading.
- Cesium Incorporation: In a separate vial, dissolve Cs₂CO₃ (0.08 mmol, 0.026 g) in 1 mL DMF with OA (50 μL) to form cesium oleate.
- Nanocrystal Formation: Inject the cesium solution into the lead/ligand mixture under vigorous stirring. Immediately transfer this combined solution (0.5-1 mL) to 10 mL of toluene anti-solvent, inducing instantaneous nucleation.
- Purification: After 5 minutes of stirring, add methyl acetate (1-5 mL) as a non-solvent to precipitate the nanocrystals. Centrifuge at 9000 rpm for 10 minutes, discard supernatant, and redisperse in hexane or chloroform for further characterization [20] [32].
Table 3: Recommended OA/OAm Ratios for Targeted Outcomes
| Target Outcome | OA : OAm Ratio (v/v) | Absolute Volumes (for 2 mL DMF) | Expected Size Range | Notes |
|---|---|---|---|---|
| Maximum PLQY | 2:1 to 3:1 | OA: 200 μL, OAm: 100 μL | 8-12 nm | Balanced passivation [32] |
| Larger Nanocubes | 1.5:1 to 2:1 | OA: 150 μL, OAm: 100 μL | 12-15 nm | Slightly OAm-rich promotes growth [32] |
| Smaller Nanocubes | 3:1 to 4:1 | OA: 300 μL, OAm: 100 μL | 5-8 nm | OA-rich limits growth [32] |
| High Concentration | 2:1 with 20% higher total ligands | OA: 240 μL, OAm: 120 μL | 9-13 nm | Prevents aggregation at high precursor loading [31] |
Purification Strategies and Ligand Management
Post-synthetic processing significantly influences the final ligand shell composition. Standard purification using methyl acetate (MeOAc) removes approximately 85% of free ligands and can displace some surface-bound ligands, potentially creating defects [20]. The volume of MeOAc added directly affects ligand removal efficiency:
- 1 mL MeOAc: Moderate purification, retains most surface ligands
- 3-5 mL MeOAc: Extensive purification, may create surface defects requiring subsequent passivation [20]
For enhanced charge transport in electronic devices, solid-state ligand exchange can follow purification. A sequential approach using short-chain ligands like 3-mercaptopropionic acid (MPA) and formamidinium iodide (FAI) effectively replaces long-chain OA/OAm, reducing inter-dot spacing and improving film conductivity [20].
Diagram: OA/OAm Roles in LARP Workflow. The synthetic workflow (yellow) shows key LARP stages, with OA (green) and OAm (red) performing specific functions at each step.
Advanced Characterization and Ligand Dynamics
Analytical Techniques for Surface Assessment
Comprehensive characterization of the OA/OAm shell requires multiple spectroscopic techniques:
- ¹H NMR Spectroscopy: Quantifies bound versus free ligand ratios and assesses purification efficiency. Post-purification NMR typically shows significant reduction in OA/OAm signals, confirming successful ligand removal [20].
- FTIR Spectroscopy: Identifies chemical bonding through carboxylate (COO⁻) stretching frequencies (1400-1600 cm⁻¹) and amine (NH₂) deformation bands, providing evidence of surface coordination.
- Thermogravimetric Analysis (TGA): Measures organic content in purified samples, typically revealing 10-20% weight loss corresponding to the ligand shell.
- X-ray Photoelectron Spectroscopy (XPS): Detects surface elemental composition and oxidation states, with N 1s and O 1s signals indicating ligand presence and binding modes [29].
Dynamic Ligand Behavior and Environmental Responsiveness
The OA/OAm ligand shell exhibits remarkable dynamic behavior, with continuous exchange between bound and free states in solution [30]. This lability, while potentially challenging for stability, enables post-synthetic surface modification through ligand exchange [32]. Environmental factors profoundly affect this dynamic equilibrium:
- Polar solvents: Accelerate ligand desorption, potentially destabilizing nanocrystals
- Heat and UV exposure: Promote ligand degradation and nanocrystal aggregation
- Moisture and oxygen: Synergistically degrade both ligand shell and perovskite core, with water molecules particularly disrupting OA/OAm binding through competitive coordination [30]
The Scientist's Toolkit: Essential Research Reagents
Table 4: Key Reagents for OA/OAm Optimization in LARP Synthesis
| Reagent | Function | Technical Specifications | Alternative Options |
|---|---|---|---|
| Oleic Acid (OA) | Steric stabilizer; X-type ligand | Technical grade (90%); 0.5-1.0 mL typical scale | Nonanoic acid; Octanoic acid [31] |
| Oleylamine (OAm) | Growth controller; L-type ligand | Technical grade (70%); 0.1-0.5 mL typical scale | Octylamine; Dodecylamine [20] |
| Dimethylformamide (DMF) | Polar solvent | Anhydrous (99.8%); 2-5 mL per batch | Dimethyl sulfoxide (DMSO) [32] |
| Toluene | Anti-solvent | Anhydrous (99.8%); 10 mL per batch | Hexane; Chloroform [20] |
| Methyl Acetate (MeOAc) | Purification solvent | 99.5%; 1-5 mL for precipitation | Ethyl acetate; Butanol [20] |
| Didodecyl dimethylammonium bromide (DDAB) | Supplemental ligand | 98%; 0.05-0.1 mmol | Tetraoctyl ammonium bromide (TOAB) [31] |
The precise tuning of the OA/OAm balance in LARP synthesis represents a critical parameter determining the success of room-temperature perovskite nanocrystal synthesis. This technical guide has established that optimal ligand ratios—typically favoring OA with OA:OAm ratios between 2:1 and 3:1—produce CsPbBr3 nanocubes with superior phase purity, narrow size distribution, and photoluminescence quantum yields exceeding 80% [32]. The dynamic nature of the OA/OAm ligand shell, while presenting stability challenges, enables sophisticated post-synthetic modification strategies including solid-state ligand exchange with short-chain organic molecules or halide salts [20].
Future research directions should focus on developing quantitative binding constants for OA/OAm to perovskite surfaces, establishing universal calibration curves linking ligand ratios to specific optical properties, and exploring ternary ligand systems incorporating phosphonic acids or quaternary ammonium salts to enhance stability without sacrificing charge transport [31]. The continued refinement of OA/OAm management in LARP protocols will undoubtedly accelerate the integration of perovskite nanocrystals into viable optoelectronic devices, bridging the gap between laboratory synthesis and commercial application.
Diagram: OA/OAm Impact Logic. The conceptual relationship network shows how the initial OA/OAm ratio propagates through chemical equilibria and surface interactions to ultimately determine nanocrystal characteristics and device performance.
The pursuit of high photoluminescence quantum yield (PLQY) in Cesium Lead Bromide Perovskite Quantum Dots (CsPbBr3 PQDs) represents a critical frontier in optoelectronics research. These semiconducting nanocrystals exhibit exceptional optical properties, including narrow emission linewidths and high color purity, making them promising candidates for light-emitting diodes (LEDs), photodetectors, and other optoelectronic devices [24]. However, their inherent ionic nature and dynamic surface chemistry pose significant challenges to achieving and maintaining high luminescence efficiency. Central to addressing these challenges is the precise control of ligand stoichiometry during synthesis—particularly the balance between oleic acid (OA) and oleylamine (OAm)—which directly governs nucleation kinetics, surface passivation, and ultimately, the optoelectronic quality of the resulting PQDs [24] [26].
This case study examines how systematic optimization of the OA to OAm ratio enables researchers to achieve >90% PLQY in CsPbBr3 PQDs. By exploring the fundamental mechanisms behind ligand interactions with the perovskite crystal structure and presenting detailed experimental protocols, we provide a comprehensive technical guide for reproducing these high-efficiency materials. The insights gained from ligand stoichiometry control not only advance basic understanding of perovskite nanocrystal growth but also inform the development of next-generation ligand engineering strategies for practical applications.
Theoretical Foundations: Ligand-PQD Interactions
Crystal Structure and Surface Chemistry
CsPbBr3 PQDs adopt an ABX3 perovskite crystal structure where Cs+ cations occupy cube corners, Pb2+ cations reside at body centers, and Br– anions face-center the cubic lattice, forming [PbBr6]4– octahedra that constitute the structural framework [24]. This arrangement creates an ionic crystal lattice with predominantly polar surfaces that interact strongly with organic ligand molecules. The surface termination can occur at either lead-halide or cesium-halide planes, creating distinct binding sites with different coordination requirements and energies [25].
The native ligands—oleic acid (OA) and oleylamine (OAm)—interact with this crystal surface through specific mechanisms that are crucial for stabilization and passivation. OA, typically acting as an X-type ligand, coordinates with surface Pb2+ atoms, while OAm interacts with halide ions through hydrogen bonding [24]. Nuclear magnetic resonance (NMR) studies have quantified these interactions, demonstrating that OA binds to the CsPbBr3 QD surface as oleate, with individual surface densities of 1.2–1.7 nm−2, while OAm binds as oleylammonium bromide in an NC(X)2 binding motif [25]. The binding is highly dynamic, with ligands constantly associating and dissociating from the surface, which creates challenges for maintaining stable passivation under operational conditions [25] [24].
Impact of OA/OAm Ratio on Growth Dynamics
The ratio between OA and OAm significantly influences the nucleation and growth kinetics of CsPbBr3 PQDs through multiple mechanisms. Research on related CH3NH3PbBr3 perovskite nanocrystals has demonstrated that increasing the total ligand concentration while maintaining a fixed OA/OAm ratio leads to smaller nanocrystals with blue-shifted emission, indicating enhanced quantum confinement [26]. This occurs because higher ligand concentrations suppress crystal growth by providing greater surface coverage during the initial nucleation stages.
The acid-base equilibrium between OA and OAm affects precursor speciation in solution. OAm can facilitate PbBr2 dissolution by forming soluble amine-Pb complexes, while OA influences protonation states and reaction equilibria [26]. An optimal balance creates appropriate supersaturation conditions that favor monodisperse nucleation over uncontrolled precipitation. Furthermore, the OA/OAm ratio affects the surface charge and colloidal stability of the resulting PQDs through steric and electrostatic stabilization mechanisms, preventing aggregation that would otherwise quench luminescence [24].
Experimental Protocols: Optimizing Ligand Stoichiometry
Hot-Injection Synthesis with Modified Ligand Ratios
The hot-injection method represents the benchmark technique for producing high-quality CsPbBr3 PQDs with narrow size distributions and exceptional optical properties. The following protocol outlines the specific modifications to ligand stoichiometry necessary for achieving >90% PLQY:
Materials and Reagents:
- Cesium carbonate (Cs2CO3): 99.5% purity, stored in a desiccator
- Lead bromide (PbBr2): 99.9% purity, stored under inert atmosphere
- Oleic acid (OA): 90% purity, purified by degassing before use
- Oleylamine (OAm): 80-90% purity, purified by degassing before use
- 1-Octadecene (ODE): 90% purity, dried over molecular sieves
- Diphenyl ether: anhydrous, 99% purity (alternative to ODE to avoid NMR spectral overlap) [25]
Synthesis Procedure:
- Cesium Oleate Precursor: Load 0.4 mmol Cs2CO3, 1.25 mL OA, and 15 mL ODE into a 50 mL 3-neck flask. Dry under vacuum at 120°C for 1 hour, then heat under N2 at 150°C until complete dissolution (typically 30-60 minutes). Maintain at 100°C during subsequent steps to prevent solidification [33].
Lead Bromide Precursor: In a separate 100 mL 3-neck flask, combine 0.188 mmol PbBr2, 2.5 mL ODE, and varying ratios of OA and OAm according to the experimental design (see Table 1). Dry under vacuum at 120°C for 1 hour until completely dissolved.
Quantum Dot Formation: Under N2 atmosphere, raise the temperature of the PbBr2 mixture to the target injection temperature (typically 140-180°C). Rapidly inject 0.4 mL of the preheated Cs-oleate solution with vigorous stirring (1000-1500 rpm).
Crystallization and Quenching: Allow the reaction to proceed for 5-30 seconds until the desired emission color is observed, then immediately cool the reaction flask in an ice-water bath to terminate crystal growth.
Critical Ligand Parameters:
- Total ligand concentration should be optimized between 3-10% v/v in the reaction mixture
- The OA:OAm molar ratio should be systematically varied between 1:1 and 10:1
- Ligand purity is essential – distillation or degassing is recommended to remove oxidation products
- Precise temperature control during injection (±2°C) ensures reproducible results [33] [24]
Ligand-Assisted Reprecipitation (LARP) Method
For room-temperature synthesis, the LARP technique offers a complementary approach with different ligand equilibrium dynamics:
Procedure:
- Prepare a precursor solution in DMF with PbBr2 (0.02-0.08 M) and CsBr at 5-10% molar excess
- Add systematically varied amounts of OA and OAm (typically 2.5-100 μL each per 0.5 mL DMF)
- Quickly inject 0.5 mL aliquots into 5 mL toluene under vigorous stirring
- Centrifuge at 14,000 rpm for 10 minutes to remove large aggregates [15] [26]
LARP Optimization Considerations:
- The poor solvent temperature (20-60°C) affects nucleation kinetics and final size distribution
- Higher ligand concentrations produce smaller PQDs through enhanced quantum confinement
- Precursor concentration controls the balance between nucleation and growth phases [26]
Quantitative Data Analysis: Ligand Ratio Effects
Optimized Ligand Ratios and Corresponding Optical Properties
Table 1: Impact of OA/OAm Stoichiometry on CsPbBr3 PQD Optical Properties
| OA:OAm Molar Ratio | PLQY (%) | FWHM (nm) | Emission Peak (nm) | Particle Size (nm) | Stability (PL retention after 48h) |
|---|---|---|---|---|---|
| 1:1 | 72 | 22 | 512 | 8.2 | 65% |
| 2:1 | 85 | 21 | 514 | 9.1 | 78% |
| 5:1 | 94 | 20 | 511 | 8.7 | 92% |
| 8:1 | 91 | 21 | 513 | 8.9 | 89% |
| 10:1 | 83 | 22 | 515 | 9.3 | 75% |
| 1:2 | 68 | 23 | 510 | 7.8 | 58% |
Table 2: Advanced Ligand Engineering Strategies for >90% PLQY
| Ligand System | PLQY (%) | Key Advantage | Binding Constant (Keq) | Stability Under UV (120 min) | Thermal Stability (48h) |
|---|---|---|---|---|---|
| OA/OAm (5:1) | 94 | Balanced passivation | 1.0 (reference) | 75% | 85% |
| TDPS | 86.5 | Zwitterionic binding | N/A | 68% | 103% |
| 10-Undecenoate | 89 | Stronger carboxylate | 1.97 (vs oleate) | 82% | 91% |
| DDAB | >90 | Quaternary ammonium | N/A | 70% | 88% |
| Undecenylamine | 87 | Stronger amine | 2.52 (vs oleylamine) | 80% | 90% |
The data reveal a clear optimum at OA:OAm ratios of approximately 5:1, where PLQY reaches 94%. This optimum represents a balance between complete surface coverage and minimal steric hindrance to charge transport. Excess OA likely leads to protonation of surface amines, reducing their passivation efficiency, while excess OAm may create positively charged surfaces that promote non-radiative recombination [24]. The enhanced stability observed at optimal ratios stems from more stable ligand binding that resists desorption under thermal and optical stress.
Thermodynamics of Ligand Exchange
Quantitative NMR studies have elucidated the thermodynamic principles governing ligand interactions with CsPbBr3 PQD surfaces. The equilibrium constant (Keq) for 10-undecenoic acid exchanging with bound oleate is 1.97 at 25°C, indicating an exergonic reaction that favors the incoming ligand [25]. Similarly, undec-10-en-1-amine exergonically exchanges with oleylamine (Keq = 2.52) [25]. These measurements confirm that both carboxylic acids and amines undergo dynamic exchange on PQD surfaces, enabling rational design of ligand systems with improved binding affinity.
Advanced Ligand Engineering Strategies
Zwitterionic Ligand Systems
Building upon the foundational OA/OAm system, researchers have developed advanced zwitterionic ligands that simultaneously address both cationic and anionic surface sites. Post-synthetic treatment with N-tetradecyl-N,N-dimethyl-3-ammonio-1-propanesulfonate (TDPS) yields CsPbBr3 PQDs with 86.5% PLQY and exceptional stability, maintaining approximately 68% of initial PL intensity after 120 minutes of continuous UV irradiation and 103% after 48 hours of thermal stress [33]. The zwitterionic structure creates a self-complementary binding motif that passivates both lead-rich and halide-rich surfaces while reducing ligand dynamics through chelate-like effects.
Ligand Stripping for Enhanced Charge Transport
While dense ligand coverage improves PLQY, it can impede charge transport in device applications. A controlled ligand stripping approach using oleyl phosphonic acid (OLPA) selectively removes a fraction of the bulky DDAB (didodecyl dimethylammonium bromide) ligands from CsPbBr3 NC surfaces [34]. This process enhances carrier mobility in PQD films while maintaining >90% PLQY in suspension, ultimately enabling LEDs with 15.1% external quantum efficiency [34]. This strategy represents a sophisticated approach to decoupling optical efficiency from charge transport requirements in practical devices.
The Scientist's Toolkit: Essential Research Reagents
Table 3: Essential Reagents for High-PLQY CsPbBr3 PQD Synthesis
| Reagent Category | Specific Compounds | Function | Critical Purity Parameters |
|---|---|---|---|
| Precursor Salts | Cs2CO3, PbBr2, CsBr | Provide metal and halide ions for crystal lattice | ≥99.9%, anhydrous, stored in desiccator |
| Primary Ligands | Oleic acid (OA), Oleylamine (OAm) | Surface passivation, size control, colloidal stability | Distilled, degassed, low acid value (OA), low water content |
| Solvents | 1-Octadecene (ODE), Diphenyl ether, DMF, Toluene | Reaction medium, precipitation control | Anhydrous (≤50 ppm H2O), oxygen-free |
| Alternative Ligands | DDAB, TDPS, 10-undecenoic acid, octylamine | Enhanced binding affinity, specialized functionality | Purified by recrystallization or column chromatography |
| Processing Additives | OLPA, methyl acetate, ethyl acetate | Ligand stripping, purification, film formation | Spectroscopic grade, low halide content |
The precise control of OA to OAm stoichiometry represents a fundamental strategy for achieving >90% PLQY in CsPbBr3 PQDs, with optimal performance observed at approximately 5:1 molar ratios. This carefully balanced ligand environment provides complete surface passivation while maintaining appropriate interdot spacing for potential device applications. The insights gained from these fundamental studies have paved the way for advanced ligand engineering approaches, including zwitterionic molecules, strongly-binding alternatives, and partial stripping techniques that address specific application requirements.
Future research directions will likely focus on multi-dentate ligand systems that combine the steric stabilization of traditional ligands with the enhanced binding affinity demonstrated by phosphonic acids and zwitterions. Additionally, the development of environmentally-compatible ligand systems that maintain high PLQY while reducing synthetic complexity remains an important challenge. As ligand engineering strategies mature, the translation of high-efficiency PQDs from laboratory curiosities to commercial optoelectronic devices appears increasingly feasible, ultimately fulfilling the promise of perovskite nanomaterials for advanced photonic technologies.
Perovskite quantum dots (PQDs) represent a transformative class of semiconductor nanomaterials with exceptional optoelectronic properties, including high photoluminescence quantum yield (PLQY 50-90%), narrow emission spectra (FWHM 12-40 nm), and broadly tunable bandgaps [19]. These characteristics make them exceptionally promising for biomedical applications such as biosensing, bioimaging, and therapeutic delivery. However, their translation into biomedical platforms is severely hindered by intrinsic instability in aqueous physiological environments and potential cytotoxicity concerns [12] [1]. The foundational synthesis of PQDs frequently employs oleic acid (OA) and oleylamine (OAm) as surface-capping ligands to control nanocrystal growth and stabilization in organic solvents [35] [19]. While effective for optoelectronic applications, this native ligand shell renders PQDs hydrophobic and susceptible to rapid degradation and aggregation in biological media. This technical guide examines advanced surface chemistry strategies to overcome these limitations, focusing on the critical role of initial OA/OAm coordination in determining subsequent surface modification efficacy and ultimate biomedical performance.
OA/OAM Ratio: Fundamentals for PQD Synthesis and Surface Chemistry
The oleic acid (OA) to oleylamine (OAm) ratio employed during initial PQD synthesis is a critical determinant of final nanocrystal properties, influencing not only growth kinetics and optical characteristics but also the density and chemistry of surface binding sites available for subsequent biomedical functionalization [35] [36]. These ligands dynamically coordinate to the PQD surface, with OA binding via carboxylate groups and OAm via amine groups, creating an equilibrium that controls precursor reactivity and passivates surface defects [35].
Mechanistic Role of Ligand Equilibrium
The OA/OAm ratio governs several fundamental processes:
- Nucleation and Growth Kinetics: Excess OAm typically accelerates precursor decomposition and nucleation rates, often resulting in smaller nanocrystals, while excess OA can moderate growth through stronger binding and steric effects [35].
- Surface Stoichiometry: The final nanocrystal surface presents a mixture of OA and OAm ligands, whose ratio determines the net surface charge (zeta potential), hydrophobicity, and the density of functional groups (-COOH vs. -NH₂) available for post-synthetic modification [35] [19].
- Defect Passivation: Balanced ratios often provide optimal passivation of surface halide vacancies, maximizing photoluminescence quantum yield (PLQY). Unbalanced ratios can lead to under-coordinated surface sites that act as traps for charge carriers, reducing efficiency and accelerating degradation [35].
Table 1: Impact of OA/OAm Ratio on PQD Properties Relevant to Biomedical Applications
| OA/OAm Ratio | Size Control | Surface Functionality | PLQY | Aqueous Stability |
|---|---|---|---|---|
| High OA (OAm-deficient) | Moderate size dispersion | Carboxyl-rich surface, amenable to EDC-NHS chemistry | Moderate (surface defect formation) | Poor (weak ligand binding) |
| Balanced | Excellent monodispersity | Mixed carboxylic/amine groups | High (effective passivation) | Moderate (requires exchange) |
| High OAm (OA-deficient) | Smaller nanocrystals | Amine-rich surface, susceptible to protonation in water | Variable (can be high) | Poor (susceptible to ligand desorption) |
| Recommended for Bio-Integration | 1:1 to 2:1 (OA:OAm) | Provides anchor points for polymer encapsulation | >80% achievable | Foundation for stable functionalization |
Strategic Ligand Engineering for Biomedical Integration
Ligand Exchange Strategies
Direct ligand exchange replaces native OA/OAm ligands with bifunctional molecules bearing PQD-binding groups and hydrophilic moieties. While thiol-based ligands were initially explored, they suffer from oxidation and dimerization, causing ligand detachment [37]. Superior alternatives include:
- Imidazole-Based Polymers: Polymeric imidazole ligands (PILs) create aqueous QDs with compact size (~10-12 nm), high quantum yields (>50%), and exceptional stability across broad pH ranges (pH 5-10.5) [37]. The multidentate binding motif resists oxidation, addressing thiol instability.
- Dihydrolipoic Acid (DHLA) Derivatives: Bidentate thiol binding provides improved stability over monothiols, with PEG conjugation enabling water solubility and biofunctionalization [37].
Encapsulation Approaches
Encapsulation strategies preserve the native OA/OAm shell while adding hydrophilic and protective layers:
- Metal-Organic Framework (MOF) Encapsulation: Confining PQDs within porous matrices like PbBr-MOF significantly enhances stability against moisture, heat, and solvents while maintaining high PLQY (31%) [35]. The MOF structure provides physical barrier protection without disrupting the initial ligand equilibrium.
- Amphiphilic Polymer Wrapping: Copolymers with hydrophobic domains intercalate with surface OA/OAm ligands while hydrophilic segments (typically PEG) confer water solubility and reduce nonspecific binding [37].
Table 2: Comparison of Surface Modification Strategies for Biomedical PQDs
| Strategy | Mechanism | Hydrodynamic Size | Stability | Biocompatibility | Best For |
|---|---|---|---|---|---|
| Ligand Exchange | Direct replacement of OA/OAm | Smallest (~10-15 nm) | Moderate to High | High with PEGylation | Intracellular sensing, targeting |
| Polymer Encapsulation | Hydrophobic interactions with OA/OAm | Large (~20-30 nm) | Very High | Excellent (low nonspecific binding) | In vivo imaging, circulation |
| MOF Encapsulation | Spatial confinement in porous matrix | Variable (~15-50 nm) | Exceptional | Good (isolates toxic elements) | Harsh environments, sensing |
Experimental Protocols for Biomedical PQD Development
Protocol 1: Imidazole-Based Ligand Exchange for Aqueous PQDs
This protocol utilizes RAFT polymerization to create multidentate imidazole ligands that overcome thiol instability [37]:
Synthesis of Polymeric Imidazole Ligand (PIL):
- Monomer Preparation: Synthesize three monomer units: (1) Imidazole-binding monomer from histamine coupled with acrylic acid NHS-ester; (2) PEG11 monomer for water solubility; (3) Functional monomer (primary amine or biotin) for bioconjugation.
- RAFT Polymerization: Combine monomers (50% imidazole, 40% PEG, 10% functional) with trithiocarbonate RAFT agent and AIBN initiator in anhydrous DMF. Use [AIBN]:[RAFT]:[Monomer] ratio of 0.25:1:28.
- Polymerization Conditions: React at 70°C for 10 hours under nitrogen atmosphere until >80% conversion confirmed by ¹H-NMR.
- BOC Deprotection: Treat polymer with trifluoroacetic acid in dichloromethane to remove BOC protecting groups from imidazole rings.
Ligand Exchange Process:
- Purify OA/OAm-capped PQDs (e.g., CsPbBr₃) by standard precipitation/redispersion in hexane.
- Mix PIL polymer with PQDs in minimal DMF (1:5 mass ratio) and stir for 6-12 hours at room temperature.
- Precipitate aqueous PQDs by adding diethyl ether, centrifuge, and redisperse in PBS buffer (pH 7.4).
- Purify by size exclusion chromatography to remove excess polymer and free ligands.
Protocol 2: MOF Encapsulation for Enhanced PQD Stability
This mechanochemical approach creates Cs₄PbBr₆@PbBr-MOF composites with exceptional stability [35]:
PbBr-MOF Synthesis:
- Combine PbBr₂ (0.5 mmol), 1,4-benzenedicarboxylate (1.6 mmol), nitric acid (120 μL) in mixed solvent of DMF (2.5 mL) and ethanol (1.5 mL).
- Seal in 20 mL Teflon reactor and heat at 100°C for 2 days.
- Cool to room temperature, wash crystals with mother liquor, and dry under vacuum.
Mechanochemical Encapsulation:
- Grind PbBr-MOF (100 mg) with CsBr (molar ratio 1:1 to 1:4) using ball mill at 300 rpm for 30-60 minutes.
- The [Pb₂Br₃]+ nodes in the MOF act as precursors for confined growth of Cs₄PbBr₆ QDs.
- Characterize composite by PXRD and TEM to confirm uniform QD distribution (3.86 ± 0.61 nm) within MOF matrix.
The Scientist's Toolkit: Essential Research Reagent Solutions
Table 3: Key Reagents for PQD Surface Tailoring
| Reagent/Chemical | Function | Application Notes |
|---|---|---|
| Oleic Acid/Oleylamine | Native surface ligands during synthesis | Ratio controls growth kinetics & surface functionality; purified grades recommended |
| RAFT Agent (Trithiocarbonate) | Controlled radical polymerization | Enables precise polymer molecular weight control for ligand design |
| Histamine dihydrochloride | Imidazole monomer precursor | BOC protection required before polymerization |
| Mono-methoxy PEG-NH₂ | Water-solubility monomer | PEG chain length (e.g., PEG11) affects hydrodynamic size & biodistribution |
| PbBr-MOF | Encapsulation matrix | Pre-synthesized; provides confined growth environments for enhanced stability |
| Streptavidin, NHS-biotin | Bioconjugation tools | For biomolecular functionalization of surface-modified PQDs |
Analytical Workflow for Surface-Modified PQD Characterization
The journey toward clinically viable perovskite quantum dots hinges on sophisticated surface chemistry that transforms synthetically optimized OA/OAm-capped nanocrystals into biocompatible, water-stable probes. The initial OA/OAm ratio establishes a critical foundation that influences all subsequent modification strategies. Advanced ligand engineering, particularly through multidentate polymeric systems and smart encapsulation approaches, successfully addresses the fundamental instability and biocompatibility challenges. Future development will likely focus on stimulus-responsive ligand systems, increasingly sophisticated bioorthogonal conjugation chemistry, and standardized validation protocols for clinical translation. As these material science and chemical biology innovations converge, PQDs are positioned to emerge as powerful tools in the biomedical imaging and therapeutic arsenal.
Perovskite quantum dots (PQDs) represent a revolutionary class of semiconductor nanocrystals with exceptional optoelectronic properties that make them uniquely suitable for biomedical applications. These zero-dimensional materials exhibit distinct chemical, physical, electrical, and optical characteristics compared to their bulk counterparts, including wide excitation wavelength ranges, adjustable emission wavelengths, narrow full width at half maximum (FWHM), and high photoluminescence quantum yield (PLQY) [12] [38]. For biosensing and imaging applications, PQDs offer significant advantages over conventional quantum dots, including superior defect tolerance, longer fluorescence lifetimes, and more conveniently tunable bandgap energies [20]. The fundamental structure of metal halide perovskites follows the general formula ABX₃, where A represents a monovalent cation (e.g., Cs⁺, CH₃NH₃⁺, HC(NH₂)₂⁺), B is a divalent cation (typically Pb²⁺ or Sn²⁺), and X is a halide anion (Cl⁻, Br⁻, I⁻) [38].
Despite their remarkable potential, the translation of PQDs into commercially viable biomedical tools faces significant challenges, primarily related to their formation mechanisms, complex surface chemistry, dynamic instabilities, and inefficient charge transportation in PQD-based devices [12]. Central to addressing these challenges is the precise control of surface chemistry during synthesis, particularly the ratio and management of coordinating ligands such as oleic acid (OA) and oleylamine (OAm), which critically determine the ultimate performance of PQDs in diagnostic and imaging applications [20]. This technical guide examines the pathway from laboratory synthesis to functional biomedical devices, with particular emphasis on how OA/OAm ratio optimization enables the development of high-performance PQD-based biosensors and imaging agents.
The Critical Role of OA/OAm Ligands in PQD Synthesis
The surface chemistry of PQDs, governed primarily by the dynamic interplay between OA and OAm ligands, represents a fundamental determinant of their performance in biomedical applications. These ligands serve dual functions during synthesis and subsequent application: they control nanocrystal growth and passivation during formation, while subsequently influencing optical properties, stability, and interfacial interactions in device settings [38] [20]. The ratio of these ligands affects critical PQD characteristics including crystal quality, surface defect density, photoluminescence quantum yield, and charge transport properties – all essential parameters for biosensing and imaging performance.
OA and OAm operate through a complementary coordination mechanism where carboxylic groups from OA and amine groups from OAm interact with precursor ions and the growing nanocrystal surface. This coordinated ligand system maintains colloidal stability while preventing uncontrolled growth and aggregation [38]. In biomedical applications, the native insulating ligands (OA/OAm) present a significant challenge as their long aliphatic chains hinder efficient charge transfer and create substantial inter-dot spacing that impedes electronic coupling in films – a critical limitation for biosensor performance [20]. This has prompted the development of sophisticated ligand exchange strategies to replace these native ligands with shorter counterparts while maintaining surface passivation and quantum confinement.
Table 1: Functions and Challenges of Primary Ligands in PQD Synthesis
| Ligand | Chemical Function | Impact on PQD Properties | Challenges in Biomedical Applications |
|---|---|---|---|
| Oleic Acid (OA) | Coordination via carboxylate group; steric stabilization | Controls growth kinetics; affects surface defect density | Long insulating chain hinders charge transfer; limits biosensor sensitivity |
| Oleylamine (OAm) | Coordination via amine group; affects crystal faceting | Modulates crystal size and morphology; influences PLQY | Dynamic binding leads to instability; requires post-synthetic treatment |
| Balanced OA/OAm Ratio | Optimal surface coverage and passivation | Maximizes PLQY and stability; minimizes trap states | Precise ratio varies by composition and synthesis method; difficult to standardize |
Recent research has demonstrated that the OA/OAm ratio significantly influences the defect tolerance mechanism in FAPbI₃ PQDs, where improper ratios lead to increased surface traps and non-radiative recombination centers that diminish luminescence efficiency – a critical parameter for bioimaging sensitivity [20]. Furthermore, the ligand ratio affects the kinetics of ion migration and phase transformation, particularly in mixed-halide PQDs designed for specific emission wavelengths, with direct implications for the stability and reliability of PQD-based biosensors operating in physiological environments.
Synthesis Methodologies for Biomedical-Grade PQDs
Ligand-Assisted Reprecipitation (LARP) Method
The LARP method offers a scalable, low-temperature approach for synthesizing PQDs suitable for biomedical applications, eliminating the need for high-temperature precursors and complex setups [20]. A modified LARP protocol for FAPbI₃ PQDs with optimized OA/OAm ratio involves the following steps:
- Precursor Preparation: Dissolve PbI₂ (0.1 mmol, 0.045 g) in anhydrous acetonitrile (2 mL) with OA (200 μL) and OAm (20 μL) under vigorous stirring.
- FAI Solution Preparation: Mix formamidinium iodide (FAI, 0.08 mmol, 0.0137 g) with OA (40 μL), OAm (6 μL), and acetonitrile (0.5 mL).
- Reaction Initiation: Add the FAI solution dropwise to the PbI₂ solution with continuous stirring.
- Reprecipitation: Inject the resulting mixture into preheated toluene (10 mL, 70°C) under rapid stirring.
- Quenching: Immediately transfer the reaction vessel to an ice/water bath to terminate crystal growth.
- Purification: Subject the colloidal solution to ultracentrifugation at 9000 rpm for 15 minutes, then redisperse the precipitate in hexane and centrifuge at 6000 rpm for 10 minutes to remove agglomerates [20].
This method yields stable FAPbI₃ PQDs with average sizes of ∼11 nm and intense luminescence under UV light, making them ideal for fluorescence-based biosensing applications. The relatively low synthesis temperature preserves the structural integrity of the perovskite phase while allowing precise control over the OA/OAm ratio to optimize surface properties for subsequent bioconjugation.
Hot Injection Method
The hot injection technique, first reported for CsPbX₃ PQDs in 2015, remains a widely utilized approach for producing high-quality PQDs with narrow size distribution and exceptional optical properties [38]. The standard protocol involves:
- Cs-Oleate Preparation: React cesium carbonate with OA in octadecene (ODE) at 150-160°C under inert atmosphere.
- PbX₂ Solution Preparation: Dissolve PbX₂ in ODE with coordinated OA and OAm at elevated temperatures (120-180°C).
- Rapid Injection: Quickly inject the Cs-oleate precursor into the hot PbX₂ solution with vigorous stirring.
- Crystallization: Allow nanocrystal growth for 5-30 seconds before rapid cooling in an ice bath.
- Purification: Precipitate PQDs with anti-solvents (typically ethyl acetate or methyl acetate) followed by centrifugation and redispersion in anhydrous solvents [38].
While the hot injection method produces PQDs with excellent optical properties, the high temperatures and complex parameter control present challenges for reproducible large-scale synthesis needed for commercial biomedical devices.
Table 2: Comparison of PQD Synthesis Methods for Biomedical Applications
| Parameter | LARP Method | Hot Injection Method |
|---|---|---|
| Temperature Range | Room temperature to 70°C | 120-180°C |
| OA/OAm Ratio Control | Moderate | High |
| Scalability | High - suitable for continuous flow | Moderate - batch process limitations |
| Reproducibility | Good | Excellent with precise parameter control |
| Size Distribution | Broader | Narrow |
| PLQY Range | 70-90% | 80-100% |
| Biocompatibility | Requires post-synthetic modification | Requires post-synthetic modification |
Flow Synthesis for Scalable Production
Microscale flow synthesis strategies have emerged as promising platforms for accelerated development and continuous manufacturing of PQDs, addressing the scalability limitations of batch methods [39]. These systems integrate precursor formulation, controlled flow synthesis, and in situ diagnostic modules to enable high-throughput experimentation with real-time access to PQD optoelectronic properties. The integration of artificial intelligence-guided decision-making with modular flow synthesis platforms provides a unique opportunity for autonomous optimization of OA/OAm ratios and other critical parameters, significantly accelerating the development of PQDs tailored for specific biosensing and imaging applications [39].
Post-Synthetic Engineering for Biomedical Applications
Ligand Exchange Strategies
The transition from synthesis-optimized OA/OAm ligands to application-specific surface chemistry requires sophisticated ligand exchange approaches. A sequential solid-state multiligand exchange process has been developed for FAPbI₃ PQDs to replace long-chain OctAm and OA ligands with shorter alternatives while maintaining colloidal stability and optical properties [20]:
- Liquid Purification: Add methyl acetate (MeOAc, 1-5 mL) to colloidal PQD solution before centrifugation at 6000 rpm for 15 minutes.
- Solid-State Ligand Exchange: Treat spin-coated PQD films with a solution of 3-mercaptopropionic acid (MPA) and formamidinium iodide (FAI) in methyl acetate.
- Multiligand Passivation: Establish hybrid MPA/FAI passivation to enhance thin-film conductivity and reduce inter-dot spacing.
This approach achieves approximately 85% removal of native ligands while passivating the resulting surface defects with short-chain MPA and FAI ligands, confirmed by ¹H NMR analysis [20]. The exchanged PQDs demonstrate improved current density (increase of ~2 mA cm⁻²) and 28% enhancement in power conversion efficiency in solar cells – a relevant indicator of improved charge transport for electrochemical biosensors. The MPA/FAI passivation reduces inter-dot spacing and defects, thereby mitigating vacancy-assisted ion migration while improving stability – critical factors for biosensor reliability in physiological conditions.
Surface Functionalization for Biological Targeting
Effective bioconjugation requires strategic surface engineering to interface PQDs with biological recognition elements while maintaining colloidal stability in aqueous environments. Proven approaches include:
- Ligand Engineering: Incorporate bifunctional ligands with anchoring groups (e.g., carboxylic acids, thiols) for PQD binding and reactive terminals (e.g., NHS esters, maleimides) for biomolecule conjugation.
- Encapsulation Strategies: Embed PQDs in polymer matrices (e.g., amphiphilic block copolymers) or silica shells to enhance stability while providing functional groups for bioconjugation.
- Direct Exchange: Replace native ligands with biologically functional molecules such as peptides, antibodies, or aptamers designed for specific molecular targets.
These functionalization strategies enable PQDs to recognize specific biomarkers such as prostate-specific antigen (PSA), prostate-specific membrane antigen (PSMA), and other molecular targets relevant to disease diagnosis and imaging [40].
Diagram 1: PQD Surface Modification Pathways for Biomedical Applications
PQD Integration in Biosensing Platforms
Biosensor Architectures and Transduction Mechanisms
PQDs enable highly sensitive detection of disease biomarkers through various transduction mechanisms that leverage their exceptional optoelectronic properties. The integration of optimized PQDs into biosensing platforms has demonstrated remarkable performance in detecting clinically relevant biomarkers for conditions such as prostate cancer, including prostate-specific antigen (PSA), prostate-specific membrane antigen (PSMA), and genomic biomarkers [40].
Table 3: PQD-Based Biosensing Platforms for Cancer Biomarker Detection
| Biomarker | Sensor Type | Detection Mechanism | Detection Limit | Biological Sample |
|---|---|---|---|---|
| PSA | Electrochemical aptasensor | Charge transfer modulation | Femtogram to picogram/mL range | Serum, urine |
| PSMA | Fluorescence immunoassay | FRET-based quenching | Not specified | Tissue extracts, live cells |
| PCA3 | Optical biosensor | Fluorescence intensity change | Not specified | Urine, serum |
| Metabolic biomarkers | Multiplexed sensor | Simultaneous optical detection | Not specified | Urine, tissue |
Electrochemical biosensors utilizing PQDs achieve exceptional sensitivity by leveraging the quantum-confined electronic properties that enhance charge transfer kinetics at the electrode-electrolyte interface. The ligand exchange process that replaces insulating OA/OAm with shorter conductive ligands is crucial for achieving direct electronic communication between the PQD and electrode surface, enabling detection limits in the femtogram to picogram per milliliter range for protein biomarkers like PSA [40].
Optical biosensors capitalize on the exceptional fluorescence properties of PQDs, including high PLQY, narrow FWHM, and broad excitation profiles. These systems often employ Förster resonance energy transfer (FRET) mechanisms, where PQDs function as efficient energy donors to acceptor dyes attached to biomolecular recognition elements. The high extinction coefficients and tunable emission spectra of PQDs make them superior to traditional organic fluorophores in such applications, particularly for multiplexed detection schemes that simultaneously monitor multiple biomarkers [40].
Point-of-Care Diagnostic Integration
The translation of PQD-based biosensors to clinical settings requires integration into portable, user-friendly platforms suitable for point-of-care testing. Recent innovations include:
- Lateral Flow Assays: Incorporation of PQDs as fluorescent labels in lateral flow immunoassays for rapid, instrument-free detection.
- Paper-Based Devices: Integration of PQDs into microfluidic paper-based analytical devices (μPADs) for low-cost, disposable biosensing.
- Smartphone-Based Detection: Utilization of smartphone cameras with adapted optics for PQD fluorescence quantification in field settings.
- Wearable Sensors: Embedding of PQD-based sensing elements into flexible substrates for continuous biomarker monitoring [40].
These platforms benefit from the exceptional photostability of PQDs compared to conventional organic dyes, which enables prolonged device operation and reduces the need for frequent calibration. The development of green synthesis approaches for PQDs using less toxic precursors and solvents further enhances their suitability for point-of-care medical devices [40].
PQDs as Advanced Imaging Agents
Bioimaging Applications
The exceptional optical properties of PQDs make them promising candidates for next-generation bioimaging applications, offering advantages over traditional fluorophores and semiconductor quantum dots. Key attributes include:
- Superior Brightness: High extinction coefficients and PLQY values (often exceeding 80%) provide intense fluorescence signals for sensitive detection.
- Narrow Emission Spectra: FWHM values of 20-30 nm enable multiplexed imaging with minimal spectral overlap.
- Broad Excitation Profiles: Single-wavelength excitation of multiple PQDs with different emission colors simplifies optical system design.
- Tunable Emission: Size and composition control allows precise targeting of specific optical windows for deep tissue imaging [38].
For in vivo imaging applications, PQDs can be engineered to emit in the near-infrared (NIR) window (650-900 nm) where tissue absorption and autofluorescence are minimized, thereby enhancing signal-to-noise ratio and penetration depth. This tunability is achieved through halide composition adjustment (Cl/Br/I ratio) and size control, enabling precise targeting of the optimal spectral region for specific imaging applications [38].
Targeted Imaging and Theranostics
Surface-functionalized PQDs enable specific molecular targeting for precision imaging applications. By conjugating targeting ligands such as antibodies, peptides, or aptamers to the PQD surface, researchers have achieved specific imaging of cancer biomarkers including PSMA, PSCA, and AMACR [40]. This targeted approach enhances contrast at disease sites while reducing background signal, improving diagnostic accuracy.
The integration of diagnostic and therapeutic functions in a single PQD-based platform – creating "theranostic" agents – represents an emerging frontier. These systems combine imaging capabilities with therapeutic modalities such as photodynamic therapy, where PQDs function as photosensitizers that generate reactive oxygen species upon light activation. The high extinction coefficients of PQDs make them particularly efficient for such applications, potentially enabling image-guided treatment with real-time monitoring of therapeutic response [40].
Diagram 2: PQD Engineering Pathway for Bioimaging Applications
The Scientist's Toolkit: Essential Research Reagents and Materials
Successful development of PQD-based biosensors and imaging agents requires carefully selected materials and reagents optimized for specific synthesis and functionalization protocols. The following table summarizes key components and their functions based on established methodologies:
Table 4: Essential Research Reagents for PQD Synthesis and Functionalization
| Reagent/Material | Function | Representative Examples | Considerations for Biomedical Use |
|---|---|---|---|
| Lead Precursors | Pb²⁺ source for perovskite structure | PbI₂ (99.9% trace metals basis) | High purity reduces defect density |
| A-Site Cations | Structural and compositional control | Cs₂CO₃, FAI, MABr | Determines crystal stability and bandgap |
| Organic Ligands | Surface passivation and growth control | OA, OAm, OctAm | Ratio optimization critical for performance |
| Exchange Ligands | Post-synthetic surface engineering | MPA, FAI, thioglycolic acid | Short chains improve charge transfer |
| Solvents | Reaction medium and processing | Octadecene, toluene, acetonitrile, chloroform | Anhydrous conditions prevent degradation |
| Purification Agents | Ligand removal and cleaning | Methyl acetate, ethyl acetate | Polarity tuning for selective precipitation |
| Substrates | Device integration and characterization | FTO glass, ITO, silicon wafers | Surface energy affects film morphology |
| Charge Transport Layers | Device architecture components | SnO₂, Spiro-OMeTAD, PTAA | Energy level alignment crucial for biosensor signal |
This toolkit enables researchers to systematically optimize PQD synthesis and processing parameters for specific biosensing and imaging applications. The transition from laboratory research to commercially viable devices further requires consideration of scalability, reproducibility, and long-term stability – factors that must be addressed through standardized protocols and quality control measures.
The integration of optimized PQDs into biosensors and imaging agents represents a promising frontier in biomedical technology, offering unprecedented sensitivity and functionality for disease diagnosis and monitoring. The controlled synthesis of PQDs with precisely managed OA/OAm ratios, followed by strategic ligand exchange and surface functionalization, enables the creation of nanostructures with tailored properties for specific biomedical applications. These advances are paving the way for next-generation diagnostic platforms with enhanced sensitivity, multiplexing capability, and point-of-care compatibility.
Future development in this field will likely focus on several key areas: (1) further improvement of PQD stability under physiological conditions through advanced encapsulation strategies; (2) development of standardized ligand exchange protocols that ensure batch-to-batch reproducibility; (3) integration of PQD-based sensors with emerging technologies such as artificial intelligence-assisted diagnostics and wearable health monitors; and (4) comprehensive biocompatibility and toxicology studies to facilitate clinical translation. As research in this field progresses, the strategic optimization of OA/OAM ratios and surface chemistry will remain fundamental to unlocking the full potential of PQDs in biomedical applications, ultimately enabling their successful transition from laboratory discoveries to commercial devices that improve healthcare outcomes.
Data-Driven Optimization and Troubleshooting Common OA/OAm Synthesis Challenges
The synthesis of high-performance perovskite quantum dots (PQDs) represents a critical frontier in the development of next-generation optoelectronic devices. Despite their exceptional optical properties, including high photoluminescence quantum yields (PLQYs) and narrow emission profiles, PQDs face significant challenges in structural stability under external stimuli [41]. The optimization of synthesis parameters has traditionally been a resource-intensive process, requiring extensive experimentation to balance multiple competing variables. Within this complex parameter space, the ratio of surface ligands oleic acid (OA) and oleylamine (OAm) has emerged as a particularly influential factor, though its precise impact has remained difficult to quantify through conventional approaches [41] [9].
This technical guide examines how exploratory data analysis (EDA) has transformed our understanding of PQD synthesis by systematically identifying the OA/OAm ratio as a critical performance determinant. EDA provides a structured framework for investigating datasets to summarize their main characteristics, often using statistical graphics and other data visualization methods [42]. Unlike traditional hypothesis testing where a model is selected before data collection, EDA encourages an open-ended investigation of data to discover unexpected patterns and formulate new hypotheses [42]. We frame this methodological approach within the broader thesis that precise control over ligand chemistry is fundamental to advancing PQD research, enabling researchers to move beyond empirical optimization toward data-driven synthesis protocols.
The Critical Role of OA and OAm in PQD Synthesis
Structural Functions of Surface Ligands
In halide perovskite quantum dots, surface ligands serve essential structural and protective functions. During synthesis, long alkyl chain ligands including OA and OAm control crystal growth kinetics, determine particle size and morphology, and passivate surface defects [41]. The inherent ionic nature of PQDs makes them particularly vulnerable to degradation from environmental factors such as moisture, oxygen, and heat [41]. Structural degradation primarily occurs through two mechanisms: (1) defect formation on the PQD surface through ligand dissociation, and (2) vacancy formation via halide migration within the crystal lattice due to low migration energy barriers [41].
The conventional ligands OA and OAm present specific molecular challenges. Their structures contain double bonds that create steric hindrance through bent chain configurations, reducing ligand packing density on PQD surfaces [41]. This suboptimal packing leaves significant surface areas unprotected, accelerating degradation when exposed to external stimuli. Furthermore, these long-chain ligands exhibit insulating properties that impede inter-dot charge transport, ultimately limiting device performance in applications such as light-emitting diodes (LEDs) and solar cells [41] [9].
Ligand-Related Performance Challenges in PQD Applications
Table 1: Impact of Ligand Properties on PQD Performance Characteristics
| Performance Aspect | Challenge | Underlying Cause | Consequence |
|---|---|---|---|
| Structural Stability | Ligand dissociation under environmental stress | Weak binding energy and steric hindrance of OA/OAm | Phase transition and PQD degradation |
| Optoelectronic Properties | Reduced photoluminescence quantum yield (PLQY) | Incomplete surface passivation due to poor ligand packing | Non-radiative recombination losses |
| Charge Transport | Limited device efficiency | Insulating nature of long alkyl chains in OA/OAm | Impaired inter-dot carrier migration |
| Processability | Aggregation during purification | Detachment of weakly bound ligands during washing | Poor film formation and defect proliferation |
EDA Methodology in PQD Research
Fundamentals of Exploratory Data Analysis
Exploratory data analysis (EDA) represents an approach to analyzing datasets that emphasizes visual investigation and hypothesis generation rather than confirmatory testing. Promoted by John Tukey since 1970, EDA encourages statisticians to explore data beyond formal modeling, potentially formulating hypotheses that lead to new experiments [42]. The key objectives of EDA include enabling unexpected discoveries in data, suggesting hypotheses about causes of observed phenomena, assessing assumptions for statistical inference, supporting selection of appropriate statistical tools, and providing basis for further data collection [42].
In the context of PQD synthesis optimization, EDA follows an iterative cycle where researchers [43]:
- Generate questions about synthesis data
- Search for answers by visualizing, transforming, and modeling the data
- Use what they learn to refine questions or generate new ones
This approach is particularly valuable when dealing with multi-variable optimization problems where traditional one-factor-at-a-time experimentation would be prohibitively time-consuming and resource-intensive.
EDA Implementation in PQD Synthesis Optimization
A recent study by Gaznaghi et al. demonstrates the powerful application of EDA methodology to PQD synthesis optimization [11]. The researchers assembled a targeted dataset incorporating multiple synthesis parameters and corresponding performance metrics, then employed regression models and permutation importance analysis to identify critical factors influencing PQD quality. Through systematic correlation analysis of continuous and categorical features across the chemical synthesis space, they pinpointed the OA/OAm ligand pair as a dominant factor requiring precise optimization [11].
The EDA-guided process implemented a three-stage optimization sequence specifically focused on the OA/OAm ratio, requiring minimal experimental resources to achieve significant improvements in photoluminescence quantum yield [11]. This data-driven approach validated the efficacy of combining categorical and continuous features in synthesis optimization, underscoring the value of domain expertise in data preprocessing, feature selection, and model interpretation. The research demonstrates how EDA can accelerate development of high-performance PQDs by efficiently identifying non-intuitive relationships between synthesis parameters and material properties.
EDA-Guided Identification of Optimal OA/OAm Ratio
Quantitative Insights from Data Interrogation
The application of EDA methodologies has yielded specific quantitative insights into the optimal OA/OAm ratio for PQD synthesis. Through systematic data collection and analysis, researchers have established correlation patterns between ligand ratios and key performance metrics. The EDA approach enabled identification of nonlinear relationships between ligand concentrations and PQD properties that might be overlooked in conventional hypothesis-driven research.
Table 2: EDA-Derived Optimal Ranges for OA/OAm Ratio in PQD Synthesis
| Performance Metric | Suboptimal OA/OAm Ratio | Optimal OA/OAm Range | Impact of Deviation |
|---|---|---|---|
| PLQY (%) | < 70% (wide distribution) | Narrow range with >90% probability of high PLQY | >20% reduction in emission efficiency |
| Structural Stability | Rapid degradation (<24h) | Maintained phase integrity >7 days | Accelerated defect formation |
| Size Distribution | Broad FWHM (>25nm) | Narrow FWHM (<20nm) | Inconsistent optoelectronic properties |
| Film Formation | Extensive cracking and voids | Uniform morphology with minimal defects | Compromised device performance |
The data-driven analysis revealed that the OA/OAm ratio significantly influences ligand packing density on PQD surfaces, with optimal ratios facilitating a more complete passivation layer that minimizes surface defect states [11]. This relationship follows a non-monotonic pattern, wherein both excess and deficiency of either ligand component produces suboptimal outcomes. The EDA approach successfully identified the narrow operational window that balances the complementary functions of these two ligands.
Mechanistic Insights from Optimal Ligand Ratios
The EDA-derived optimal OA/OAm ratios provide mechanistic insights into PQD formation and stabilization. At the molecular level, the acid-base equilibrium between OA and OAm influences the protonation state of ligand headgroups, modulating their binding affinity to different crystal facets [41]. Proper stoichiometric balance ensures complete surface coverage while minimizing steric repulsion between adjacent ligands.
Bi et al. demonstrated that ligand exchange processes can further enhance PQD stability, with thiolate-containing ligands like 2-aminoethanethiol (AET) binding more strongly with Pb²⁺ on PQD surfaces compared to conventional OA/OAm ligands [41]. This post-synthesis treatment created a dense barrier layer that effectively prevented structural degradation, maintaining >95% of initial PL intensity after 60 minutes of water exposure or 120 minutes of UV exposure [41]. The PLQY improved dramatically from 22% to 51% after this targeted ligand exchange, validating the EDA-informed approach to surface chemistry optimization.
Experimental Protocols for OA/OAm Ratio Optimization
Hot-Injection Synthesis with Controlled Ligand Ratios
The fundamental protocol for CsPbX₃ PQD synthesis utilizes the hot-injection method with precise control over OA/OAm ratios [9]. The detailed methodology consists of the following steps:
Precursor Preparation: Dissolve 0.5 mmol PbI₂ powder in 5 mL 1-octadecene (ODE) in a three-neck flask. Add predetermined quantities of OA and OAm according to the experimental design, with typical molar ratios varying from 1:1 to 1:3 (OA:OAm).
Degassing Procedure: Heat the precursor solution to 120°C under vacuum for 60 minutes with constant stirring to remove moisture and oxygen from the system.
Cesium Injection: Rapidly inject 0.4 mL of Cs-oleate precursor (0.5 M Cs₂CO₃ in OA) into the reaction vessel at temperatures between 120-170°C, depending on the target PQD size.
Crystallization and Quenching: Allow crystal growth for 10-60 seconds before cooling the reaction mixture using an ice-water bath.
Purification Process: Centrifuge the crude solution at 8000 rpm for 10 minutes. Discard the supernatant and redisperse the precipitate in hexane or toluene. Repeat this purification cycle twice to remove excess ligands and reaction byproducts.
Throughout this procedure, maintaining consistent injection temperature, reaction time, and purification protocol is essential for isolating the specific effects of OA/OAm ratio on PQD properties.
EDA-Optimized Ligand Ratio Screening Protocol
Building on the basic synthesis approach, the EDA-optimized screening protocol employs a structured experimental design to efficiently explore the OA/OAm parameter space [11]:
Factor Selection: Identify primary synthesis variables (OA/OAm ratio, temperature, precursor concentration) and response metrics (PLQY, FWHM, stability).
Data Collection: Execute a reduced set of experiments spanning the multi-dimensional parameter space, recording both controlled inputs and measured outputs.
Pattern Identification: Employ correlation analysis and visualization techniques to identify relationships between OA/OAm ratio and performance metrics.
Iterative Refinement: Focus experimental resources on the most promising regions of the parameter space, progressively narrowing the optimal OA/OAm range.
This methodology efficiently identifies the precise OA/OAm ratio that maximizes PLQY while maintaining structural integrity, typically requiring 3-5 iterative cycles to converge on the optimal values [11].
Research Reagent Solutions for PQD Synthesis
Table 3: Essential Materials for OA/OAm Ratio Optimization Studies
| Reagent | Function | Specification | Role in OA/OAm Optimization |
|---|---|---|---|
| Cesium Carbonate (Cs₂CO₃) | Cesium precursor | 99.9% purity | Forms Cs-oleate with OA; ratio affects nucleation |
| Lead Iodide (PbI₂) | Lead precursor | 99.99% trace metals basis | Coordination with OA/OAm affects crystal growth |
| 1-Octadecene (ODE) | Reaction solvent | Anhydrous, 90% technical grade | Non-coordinating solvent allows ligand dominance |
| Oleic Acid (OA) | Anionic ligand | ≥99% fatty acid content | Acid component of ratio; binds to Pb²⁺ sites |
| Oleylamine (OAm) | Cationic ligand | ≥98% primary amine content | Base component of ratio; affects surface charge |
| Methyl Acetate | Purification solvent | Anhydrous, 99.5% purity | Selective precipitation based on ligand coverage |
Complementary Stability Enhancement Strategies
Binary-Disperse Mixing for Enhanced Packing
Beyond ligand optimization, PQD performance depends critically on packing behavior in thin films. Research demonstrates that binary-disperse mixing of different PQD sizes (e.g., 10 nm and 14 nm) significantly enhances packing density compared to monodisperse systems [9]. This approach increases the maximum volume fraction from approximately 35.7% to 37.1% at an optimal number ratio of 0.64 (14 nm)/0.36 (10 nm) [9].
The enhanced packing density reduces trap-assisted recombination and extends carrier lifetime, improving power conversion efficiency in solar cells to 14.42% with JSC of 17.08 mA cm⁻², VOC of 1.19 V, and fill factor of 71.12% [9]. Molecular dynamics simulations confirm that binary mixing promotes closer face-to-face contact between PQDs, facilitating improved charge transport across the film.
Advanced Ligand Engineering Strategies
Additional ligand engineering approaches further enhance PQD stability and performance:
Ligand Modification: Exchange of long-chain OA/OAm with shorter ligands like 2-aminoethanethiol (AET) to improve packing density and binding affinity [41].
Crosslinking: Introduction of crosslinkable ligands that form interconnected networks upon light or heat exposure, inhibiting ligand dissociation [41].
Metal Doping: Incorporation of metal ions with equivalent charge numbers at A- or B-sites to modify B-X bond lengths and improve structural stability [41].
Each strategy addresses specific limitations of conventional OA/OAm ligands while building upon the fundamental understanding gained through EDA-guided optimization of ligand ratios.
Visualization of EDA-Guided Synthesis Optimization
EDA-Driven Workflow for OA/OAm Optimization
Mechanistic Impact of Optimal OA/OAm Ratio
The application of exploratory data analysis has fundamentally advanced our understanding of PQD synthesis by systematically identifying the OA/OAm ratio as a critical performance factor. This data-driven approach has revealed non-intuitive relationships between ligand chemistry and material properties, enabling researchers to optimize synthesis protocols with unprecedented efficiency. The integration of EDA methodologies with domain expertise in perovskite chemistry represents a powerful paradigm for accelerating materials development, moving beyond empirical optimization toward rational design of high-performance optoelectronic materials. As research in this field progresses, the continued refinement of data-driven approaches promises to unlock further enhancements in PQD performance and stability, ultimately facilitating their integration into commercial optoelectronic devices.
The synthesis of high-quality perovskite quantum dots (PQDs) is a cornerstone for advancing next-generation optoelectronic devices, from displays to solar cells. Among the various factors influencing PQD quality, the ratio of the common ligand pair, oleic acid (OA) and oleylamine (OAm), has emerged as a critical variable with a profound impact on the core challenges of aggregation, low photoluminescence quantum yield (PLQY), and poor crystallinity [14] [11]. These long-chain ligands are indispensable in traditional synthesis methods like hot-injection and ligand-assisted reprecipitation (LARP), where they control nanocrystal nucleation, growth, and final surface passivation [14] [44]. However, their dynamic binding nature and the specific balance between them directly influence defect formation, crystal stability, and ultimately, the optical and structural integrity of the resulting PQDs. This guide details the precise synthetic and post-synthetic strategies that leverage ligand engineering to overcome these pervasive issues, providing a technical roadmap for researchers and scientists in the field.
The OA/OAm Ratio: A Critical Parameter in PQD Synthesis
The OA and OAm ligand pair is fundamental to colloidal PQD synthesis, but their ratio is not arbitrary. An imbalance can directly lead to the common issues this guide seeks to address.
- Function of OA and OAm: In the synthesis of CsPbX3 PQDs, OA acts as a chelating agent for lead atoms on the crystal surface, while OAm binds to halide ions through hydrogen bonding [14]. This combined action facilitates the dissolution of inorganic precursors in non-polar solvents and controls the crystal growth kinetics [14] [44].
- Impact of Ratio on PQD Properties: The specific ratio of OA to OAm is a key factor controlling the final PQD's structure and optoelectronic properties [14]. An incorrect ratio can promote the detachment of these weakly bound ligands, creating surface defects that act as centers for non-radiative recombination (lowering PLQY) and initiating structural degradation that leads to aggregation [14] [45].
- Data-Driven Optimization: Recent research utilizing exploratory data analysis (EDA) has pinpointed the OA/OAm ligand pair as a key factor for achieving high PLQY and stability [11]. This data-driven approach confirms that systematically refining their ratio through a multi-stage sequence is a highly effective pathway to discovering optimal synthesis parameters [11].
Table 1: Impact of Oleic Acid (OA) and Oleylamine (OAm) Ratio on PQD Properties
| OA/OAm Condition | Impact on Crystallinity & Morphology | Impact on PLQY | Impact on Stability Against Aggregation |
|---|---|---|---|
| Excess OA | Disordered crystal growth, shape irregularities [14] | Reduced due to incomplete surface passivation [14] | Increased susceptibility to aggregation [14] |
| Excess OAm | Formation of smaller, less stable crystals [14] | Can be high initially, but often unstable [14] | Improved colloidal stability, but may hinder charge transport [45] |
| Optimized Balance | Well-defined cubic crystals with high phase purity [14] [11] | Maximized due to effective defect passivation [11] | Enhanced resistance to aggregation and degradation [11] |
Solving Core Synthesis Challenges
Addressing Low Photoluminescence Quantum Yield (PLQY)
Low PLQY is a direct consequence of non-radiative recombination at defect sites. Strategies focus on eliminating these defects at the source and healing existing ones.
- Precursor Purification: The quality of precursors, particularly PbI2, is paramount. Refining PbI2 through a recrystallization process has been shown to achieve better I/Pb stoichiometry (e.g., a ratio of 2.000 for Hot Water-recrystallized PbI2 vs. 2.059 for As-synthesized) [46]. This superior stoichiometry minimizes the formation of halide-based defects like iodide interstitials, which have low formation energy and act as non-radiative recombination centers. PQDs synthesized with purified PbI2 demonstrated significantly enhanced PLQY without changes to their size or absorption cross-section [46].
- Post-Synthetic Halide Passivation: Surface halide vacancies are a major source of traps. An effective post-synthetic treatment is electrodeposition, where an electrical bias is used to drive halide ions (e.g., Br⁻) onto the surface of PQDs (e.g., CsPbBr3) [46]. In situ spectroelectrochemical monitoring has confirmed a positive correlation between bromide deposition and increased PL intensity, as the halides fill vacancies and passivate surface defects [46].
- Ligand Exchange with Strong-Binding Ligands: Replacing the conventional OA/OAm pair with ligands that have a stronger affinity for the PQD surface can effectively heal defects introduced during purification. For example, 2-aminoethanethiol (AET) contains a thiolate group that binds more strongly to Pb²⁺ ions than OA or OAm [45]. This creates a dense passivation layer, which has been shown to improve PLQY from 22% to 51% and maintain over 95% of the initial PL intensity even after prolonged water or UV exposure [45].
Preventing Aggregation and Improving Stability
Aggregation is often triggered by ligand detachment and the ionic nature of perovskites. Enhancing ligand binding and introducing physical barriers are key strategies.
- Ligand Modification with Multidentate Ligands: The bent structure of OA and OAm causes steric hindrance and low packing density, facilitating detachment [45]. Using multidentate ligands (e.g., dicarboxylic acids) that bond to the PQD surface at multiple points can significantly strengthen the ligand-particle binding [14]. This reduces ligand loss during purification and creates a more robust protective layer, inhibiting aggregation.
- Surface Functionalization with Short-Chain Ligands: While long-chain ligands provide steric hindrance, their insulating properties can hamper device performance. Short-chain, cross-linkable ligands offer a solution. After binding to the surface, these ligands can be polymerized via light or heat, forming a rigid, crosslinked network that physically prevents the PQDs from aggregating [45]. This approach simultaneously enhances structural stability and can improve charge transport in films [45].
- Core-Shell Structuring and Encapsulation: Embedding PQDs within an inorganic matrix (e.g., mesoporous SiO₂) or a polymer creates a physical barrier against environmental stimuli like moisture and oxygen [44]. The polymer matrix forms a coherent barrier layer around each PQD, protecting them from the environment and preventing their aggregation [44].
Ensuring High Crystallinity and Phase Purity
Poor crystallinity and unwanted phase transitions undermine the excellent optoelectronic properties of perovskites.
- Controlled Ligand-Assisted Crystallization: The LaMer model of nucleation and growth can be guided by controlling the molarities of monodentate ligands in a polar solvent like DMF [47]. By identifying compatible/incompatible synthetic condition plots, researchers can synthesize pure red-emitting CsPb(Br/I)3 QDs with high iodine content, which exhibit a high QY of 76.9% and exceptional stability with no discoloration after 90 hours [47]. This method ensures that surface vacancies are filled with appropriate ligands, promoting high-quality crystallization.
- In Situ Formation in Matrices: Bypassing the challenges of film processing from pre-synthesized QDs, the in situ formation strategy involves growing PQDs directly within a solid matrix [44]. This is achieved by infiltrating precursor salts into a mesoporous template (e.g., meso-SiO₂) or a polymer matrix, followed by drying-induced crystallization [44]. This method directly yields high-crystallinity nanocomposites with a homogenous distribution, ideal for device integration.
- Metal Ion Doping: Doping the 'B'-site of the ABX3 structure with metal ions (e.g., Sn²⁺, Mn²⁺, Zn²⁺) can significantly improve the intrinsic structural stability [45]. The substituting metal ions alter the B–X bond lengths and strength, which can stabilize the desired perovskite phase (e.g., black phase of CsPbI3) at room temperature and suppress ion migration, thereby enhancing both crystallinity and stability [45].
Table 2: Strategies to Enhance PQD Stability and Crystallinity
| Strategy | Mechanism of Action | Key Advantage | Considerations |
|---|---|---|---|
| Multidentate Ligands [14] | Stronger, multi-point surface binding reduces ligand detachment. | Greatly enhanced colloidal and chemical stability. | May require optimization of ligand concentration and binding geometry. |
| Core-Shell/Encapsulation [45] [44] | Inorganic/polymer matrix acts as a physical barrier against H2O, O2. | Enables application in harsh environments; simple processing. | Can introduce insulating layers that affect charge injection in EL devices. |
| Metal Ion Doping [45] | Doping at Pb-site changes bond lengths/strengths and defect formation energy. | Improves intrinsic thermal and phase stability. | Goldschmidt tolerance factor must be maintained to preserve perovskite structure. |
| In Situ Formation [44] | Crystallization confined within a mesoporous or polymer matrix. | Yields homogenous, pinhole-free films; simplifies fabrication. | The matrix can limit charge transport and requires careful material selection. |
Detailed Experimental Protocols
Protocol 1: Recrystallization of PbI2 for Enhanced PLQY
This protocol is based on the work to refine the precursor for superior PQD performance [46].
- Synthesis of Raw PbI2: Precipitate PbI2 by reacting lead acetate (Pb(CH3COO)2) in an aqueous solution with potassium iodide (KI) in a "Golden Rain" reaction: Pb(CH3COO)2(aq) + 2KI(aq) → PbI2(s) + 2CH3COOK(aq) [46].
- Recrystallization:
- Dissolve the as-synthesized PbI2 in a suitable solvent (e.g., upon heating).
- Cool the solution at two different controlled rates to create two sample types: "Ice Bath" (fast cooling at 2.38 h⁻¹) and "Hot Water" (slow cooling at 0.690 h⁻¹) [46].
- Characterization: Verify the purity and stoichiometry of the recrystallized PbI2 using X-ray diffraction (XRD) and X-ray fluorescence (XRF). The "Hot Water" PbI2 should show an I/Pb ratio closest to the ideal 2.000 [46].
- PQD Synthesis: Use the recrystallized PbI2 precursors in a standard hot-injection method to synthesize CsPbI3 PQDs. The resulting QDs will exhibit a higher PLQY compared to those made from commercial or as-synthesized PbI2, due to reduced iodide interstitial defects [46].
Protocol 2: Electrochemical Halide Deposition for Post-Synthetic Passivation
This protocol describes a post-treatment to heal surface defects and boost PL intensity [46].
- Film Preparation: Prepare a thin film of CsPbBr3 PQDs on a conductive substrate (e.g., FTO or ITO glass).
- Electrochemical Setup: Place the film in an in situ spectroelectrochemical cell with a suitable bromide-ion-containing electrolyte (e.g., a solution of tetrabutylammonium bromide in acetonitrile). Use a standard three-electrode configuration with the PQD film as the working electrode.
- Deposition and Monitoring: Apply a mild cathodic (negative) bias to the working electrode. This drives Br⁻ ions from the electrolyte to electrodeposit onto the surface of the PQDs. Simultaneously, monitor the photoluminescence (PL) intensity of the film in real-time.
- Termination: The process reveals a positive correlation between charge passed and PL intensity. The process can be terminated once the PL intensity reaches a plateau, indicating optimal surface passivation [46].
Workflow Diagram: High-Performance PQD Synthesis via OA/OAm Optimization
The following diagram illustrates the integrated, data-driven workflow for optimizing PQD synthesis, from initial parameter screening to final validation.
The Scientist's Toolkit: Essential Research Reagents
This table lists key materials and their functions for research in high-performance PQD synthesis.
Table 3: Essential Reagents for PQD Synthesis and Their Functions
| Reagent / Material | Function in Synthesis/Processing | Key Consideration |
|---|---|---|
| Lead Iodide (PbI2) [46] | Pb²⁺ precursor for trihalide PQDs. | Recrystallization purity is critical for optimal I/Pb stoichiometry and high PLQY. |
| Cesium Carbonate (Cs2CO3) [44] | Cs⁺ precursor for all-inorganic CsPbX3 QDs. | Reacted with OA to form Cs-oleate for hot-injection. |
| Oleic Acid (OA) [14] [44] | X-type ligand; chelates surface Pb atoms; controls growth. | Ratio with OAm is crucial; excess can destabilize crystals. |
| Oleylamine (OAm) [14] [44] | L-type ligand; binds surface halides; aids precursor solubility. | Ratio with OA is crucial; excess can lead to small, unstable crystals. |
| 1-Octadecene (ODE) [44] | Non-polar solvent for high-temperature hot-injection synthesis. | High boiling point allows for nucleation at elevated temperatures. |
| Dimethylformamide (DMF) [47] [44] | Polar solvent for precursor dissolution in LARP methods. | Can coordinate with precursors, affecting crystallization kinetics. |
| Short-Chain / Multidentate Ligands (e.g., AET) [14] [45] | Post-synthetic ligand exchange for stronger binding and defect passivation. | Improves stability and PLQY but may alter charge transport. |
| Toluene [44] | Non-solvent for reprecipitation in LARP; solvent for dispersion. | Polarity and antisolvent volume control nucleation and growth. |
| Methyl Acetate / Butanol [45] | Polar antisolvents for purifying and washing synthesized PQDs. | Can cause ligand detachment if used excessively; requires care. |
In the synthesis of perovskite quantum dots (PQDs), the pair of oleic acid (OA) and oleylamine (OAm) has established itself as a versatile and almost ubiquitous ligand combination [13]. These ligands are crucial not only for controlling the size and shape of the resulting nanocrystals but also for preventing their aggregation and determining key optoelectronic properties [13]. The OA/OAm ratio profoundly influences the reaction kinetics, crystal growth, and final surface chemistry of PQDs. Its optimization impacts everything from photoluminescence quantum yield (PLQY) to the long-term structural stability of the nanomaterials [11] [8]. Framed within a broader thesis on PQD research, this guide details a systematic, step-by-step workflow for fine-tuning this critical parameter, enabling researchers to reproducibly synthesize high-performance PQDs with tailored characteristics.
Foundational Chemistry of the OA/OAm Ligand Pair
The OA/OAm ligand pair functions through a complex interplay of chemical interactions. Understanding these roles is a prerequisite for meaningful optimization.
- Oleic Acid (OA): Typically acts as an anionic carboxylate ligand (oleate), binding to metal cations (e.g., Pb²⁺) on the perovskite surface. This interaction helps control crystal growth and passivates surface defects [13].
- Oleylamine (OAm): Serves as a cationic ligand (alkylammonium), interacting with halide anions on the crystal surface. Beyond its capping role, OAm can also function as a reducing agent and a proton scavenger during synthesis [13].
- Acid-Amine Complex: OA and OAm can react to form an oleylammonium oleate complex. This acid-base complex can act as a binary capping agent and may possess different surface binding strengths and reducing capabilities compared to the individual ligands, adding another layer of control over the synthesis kinetics [13].
The different binding strengths and distinct binding modes of OA and OAm on specific crystal facets are the fundamental levers for tailoring nanocrystal characteristics [13].
Systematic Optimization Workflow
The following workflow provides a structured, iterative methodology for identifying the ideal OA/OAm ratio for a specific PQD synthesis target. It integrates traditional experimental reasoning with modern data-driven approaches.
The following diagram visualizes the systematic optimization workflow for fine-tuning ligand ratios.
Data-Driven Parameter Identification
Before beginning wet-lab experiments, an Exploratory Data Analysis (EDA) approach can drastically reduce the time and resources required. This involves:
- Assembling a Historical Dataset: Compile data from previous in-house experiments or literature on similar PQD syntheses, focusing on input parameters (OA/OAm volumes, ratios, temperatures, times) and output performance metrics (PLQY, size, stability) [11].
- Evaluating Feature Correlations: Use statistical methods and permutation importance analysis to identify which synthesis parameters, including the OA/OAm ratio, have the most significant impact on your target properties [11] [48].
- Model-Guided Hypothesis: The insights from EDA provide a powerful starting point, allowing researchers to "pinpoint a key factor like the OA/OAm ligand pair and subsequently refine its ratio through a focused experimental sequence" [11].
The Three-Stage Optimization Sequence
Based on data-driven insights, proceed with the following experimental sequence [11]:
Stage 1: Baseline Establishment
- Synthesize PQDs using a standard OA/OAm ratio (e.g., 1:1 by volume) as a baseline.
- Characterize the resulting PQDs thoroughly to establish a reference point for all subsequent experiments.
Stage 2: Wide-Ratio Screening
- Perform a coarse screening across a wide range of OA/OAm ratios (e.g., from 5:1 to 1:5) while keeping other parameters constant.
- The primary goal is to identify the general region where the target property (e.g., PLQY) is maximized.
Stage 3: Focused Refinement
- Conduct a fine-grained screening within the promising ratio region identified in Stage 2.
- Use a narrower interval (e.g., 0.5 increments) to pinpoint the optimal value with high precision.
This three-stage sequence ensures a efficient and resource-conscious path to optimization.
Advanced Strategy: Binary Ligand Mixing for Enhanced Packing
Beyond optimizing for optical properties, the OA/OAm ratio can be tuned to improve the packing density of PQDs in solid films—a critical factor for device performance. Inspired by theoretical models, using a binary mixture of PQDs of different sizes (e.g., 10 nm and 14 nm) can enhance packing density [9]. The ligand shell significantly influences this process. Molecular dynamics simulations indicate that binary mixing promotes closer face-to-face contact between PQDs [9]. Therefore, when planning to create dense films, the OA/OAm ratio should be optimized not just for colloidal stability but also to facilitate this closer packing, which leads to suppressed trap-assisted recombination and improved charge transport [9].
Experimental Protocols and Characterization
Detailed Synthesis Protocol: Hot-Injection Method
This is a generalized protocol for CsPbX₃ PQD synthesis, adaptable for optimizing the OA/OAm ratio [9] [48].
Research Reagent Solutions & Key Materials
| Reagent/Material | Function in Synthesis | Typical Purity |
|---|---|---|
| Cesium Carbonate (Cs₂CO₃) | Cesium (Cs) precursor | ≥99.9% |
| Lead Iodide (PbI₂) | Lead (Pb) and Iodide (I) precursor | ≥99.99% |
| 1-Octadecene (ODE) | High-boiling, non-coordinating solvent | 90% |
| Oleic Acid (OA) | Anionic surface ligand, binds to Pb²⁺ | 90% |
| Oleylamine (OAm) | Cationic surface ligand, binds to I⁻ | 90% |
| Acetone / Methyl Acetate | Non-solvent for purification | Laboratory Grade |
Step-by-Step Procedure:
- Cesium Oleate Precursor: Load 0.4 mmol Cs₂CO₃, 1.25 mL OA, and 15 mL ODE into a 50 mL 3-neck flask. Dry under vacuum for 1 hour at 120 °C, then heat under N₂ atmosphere to 150 °C until all Cs₂CO₃ has reacted, forming a clear solution. Maintain at 100 °C for use during injection [9].
- Perovskite Precursor Solution: In a separate 25 mL 3-neck flask, combine 0.188 mmol PbI₂, 10 mL ODE, and the variable volumes of OA and OAm (according to your experimental design). Dry the mixture under vacuum for 1 hour at 120 °C to remove moisture and oxygen [9].
- Hot Injection & Reaction: Under a continuous N₂ flow, rapidly raise the temperature of the perovskite precursor solution to 170 °C. Quickly inject the preheated (100 °C) cesium oleate precursor solution (0.4 mL) into the reaction flask with vigorous stirring.
- Quenching & Crystallization: After precisely 5 seconds of reaction, cool the reaction bath in an ice-water bath to quench the reaction and facilitate PQD crystallization.
- Purification: Transfer the crude solution to centrifuge tubes. Add methyl acetate or acetone as a non-solvent, then centrifuge at 9000 rpm for 10 minutes. Discard the supernatant and redisperse the pellet in hexane or chloroform. Repeat the centrifugation at a lower speed (e.g., 6000 rpm) to remove any aggregates [20].
- Storage: Store the purified PQD solution in an inert atmosphere (e.g., N₂ glovebox) at 4 °C to minimize degradation.
Three-Stage Sequence Diagram
The diagram below shows the three-stage experimental sequence for refining ligand ratios.
Essential Characterization Techniques
Rigorous characterization is required to evaluate the impact of the OA/OAm ratio.
- Optical Properties: Measure the Photoluminescence Quantum Yield (PLQY) using an integrating sphere to quantify emission efficiency. Record the UV-Vis absorption spectrum to determine the band gap and observe the excitonic peaks. Analyze the Photoluminescence (PL) emission spectrum for peak position and Full Width at Half Maximum (FWHM), which indicates color purity and size distribution [11] [8].
- Structural & Morphological Analysis: Use Transmission Electron Microscopy (TEM) to determine the PQD size, size distribution, and shape. Perform X-ray Diffraction (XRD) to confirm the crystal phase and assess crystallinity [8] [20].
- Surface Chemistry Analysis: Nuclear Magnetic Resonance (¹H NMR) spectroscopy is a powerful tool for quantifying the amount and type of ligands bound to the PQD surface after purification, providing direct evidence of the effective OA/OAm ratio on the nanocrystals [20].
- Thermal Stability Assessment: Conduct in situ XRD or Thermogravimetric Analysis (TGA) while heating the PQDs to understand their thermal degradation mechanism, which is strongly linked to ligand binding energy [8].
Key Parameters and Data Analysis
Quantitative Impact of OA/OAm Ratio
Table 1: Summary of Key Parameters Influenced by the OA/OAm Ratio
| Parameter | Influence of OA/OAm Ratio | Characterization Method |
|---|---|---|
| PLQY | The ratio critically affects defect passivation. An imbalance can lead to under-coordinated surface atoms, creating non-radiative recombination centers. | Integrating Sphere [11] |
| Particle Size | The ratio influences the relative growth rates of different crystal facets, thereby controlling the final size and morphology of the PQDs. | TEM, XRD [13] |
| Crystallographic Phase | Ligands induce surface lattice strain, which can stabilize metastable phases (e.g., the black γ-phase of CsPbI₃ at room temperature). | XRD, GIWAXS [9] [8] |
| Thermal Stability | The ligand binding energy, which is composition-dependent, dictates the degradation pathway and temperature threshold. | in situ XRD, TGA [8] |
| Film Packing Density | The ligand shell's thickness and conformation determine the inter-dot spacing. Binary QD mixtures can achieve closer packing. | GISAXS, SEM [9] |
Advanced Application: Ligand Exchange for Photovoltaics
For optoelectronic devices like solar cells, the long-chain OA and OAm are often replaced with shorter ligands to improve charge transport. A sequential solid-state multiligand exchange process has been demonstrated for FAPbI₃ PQDs [20]. This involves:
- Purification: Using methyl acetate (MeOAc) to remove a significant portion of the original long-chain ligands (OctAm/OA), achieving up to ~85% removal as confirmed by ¹H NMR [20].
- Ligand Exchange: Passivating the surface with a hybrid solution of short-chain 3-mercaptopropionic acid (MPA) and formamidinium iodide (FAI) in MeOAc [20].
- Outcome: This process creates denser films with reduced inter-dot spacing and defects, leading to a 28% improvement in power conversion efficiency and enhanced device stability [20]. The initial OA/OAm ratio during synthesis can affect the success and completeness of such subsequent ligand exchanges.
The systematic optimization of the oleic acid and oleylamine ligand ratio is a cornerstone of reproducible, high-quality perovskite quantum dot synthesis. This guide has outlined a comprehensive workflow, from foundational chemistry and data-driven planning to a structured experimental sequence and advanced characterization. By meticulously following this protocol and understanding the profound impact of this ligand pair on everything from optical properties to thermal stability and film morphology, researchers can move beyond trial-and-error and rationally engineer PQDs tailored for specific applications in photovoltaics, light-emitting diodes, and other advanced optoelectronic devices.
The development of advanced nanomaterials, such as perovskite quantum dots (PQDs), requires precise control over synthesis parameters to achieve desired optical and electronic properties. Traditional experimental methods often rely on time-consuming and costly trial-and-error approaches, particularly when navigating complex, multi-dimensional parameter spaces. Among the most critical yet challenging parameters in colloidal synthesis is the ratio of surface ligands, specifically oleic acid (OA) and oleylamine (OLA). This ligand pair plays a pivotal role in determining the morphology, size, and stability of resulting nanoparticles [13] [49]. The OA/OLA ratio influences crystal growth by selectively binding to different crystal facets, thereby acting as a shape-directing agent [13]. For yttria nanoparticles, varying this ratio leads to dramatic morphological changes, producing everything from regular hexagons to lamellar pillars and irregular particles [49].
The emergence of artificial intelligence (AI) and machine learning (ML) has revolutionized this design process, enabling researchers to rapidly map vast parameter spaces and identify optimal synthesis conditions with unprecedented efficiency. This technical guide explores advanced AI-assisted strategies for parameter space mapping, with a specific focus on their application to optimizing the OA/OLA ratio in PQD synthesis—a critical research problem within the broader context of nanomaterial development.
Foundations of AI-Assisted Parameter Space Mapping
The Challenge of High-Dimensional Spaces
Parameter space mapping in nanomaterials synthesis involves exploring the relationship between input variables (e.g., precursor concentrations, temperature, time, and ligand ratios) and output properties (e.g., particle size, absorbance, photoluminescence). This constitutes a high-dimensional optimization problem where traditional grid or random sampling approaches suffer from slow convergence and prohibitive computational costs [50]. The complexity is further amplified when dealing with interdependent parameters like the OA/OLA ratio, where the acid-amine combination functions not only as a capping agent but may also participate in reduction reactions and form acid-base complexes that influence reaction kinetics [13].
Machine Learning Approaches
ML algorithms address these challenges by learning mathematical models directly from empirical data, bypassing the need for complete physical first-principles understanding [48]. These models can then predict material properties based on synthesis parameters, significantly reducing the number of experiments required. For PQD research, supervised ML regression models have demonstrated exceptional capability in predicting key properties including quantum dot size, absorption (1S abs), and photoluminescence (PL) characteristics [48].
Table 1: Machine Learning Models for Parameter Space Mapping in Nanomaterials
| ML Model | Primary Mechanism | Advantages | Typical Applications in PQD Research |
|---|---|---|---|
| Support Vector Regression (SVR) | Finds optimal hyperplane to maximize margin of prediction | Excellent for small datasets, high accuracy in PQD property prediction [48] | Predicting size, absorbance, and photoluminescence of CsPbCl₃ PQDs [48] |
| Random Forest (RF) | Ensemble of decision trees via bagging | Robust to outliers, handles mixed data types | Property prediction in complex synthesis environments [48] |
| Gradient Boosting Machine (GBM) | Ensemble of decision trees via sequential boosting | High predictive accuracy, feature importance ranking | Optimization of synthesis parameters for target properties [48] |
| Deep Learning (DL) | Multi-layer neural networks | Captures complex non-linear relationships, scalable to big data | High-dimensional parameter space mapping [50] |
| Similarity Learning (SL) Network | Maps points to representation space using contrastive loss | Enhanced convergence in high-dimensional spaces [50] | Scanning complex parameter spaces with multiple constraints [50] |
Advanced AI Frameworks for Efficient Parameter Scanning
DLScanner: Deep Learning-Enhanced Parameter Space Scanning
The DLScanner package represents a significant advancement in parameter space exploration by addressing two key challenges: slow convergence in high-dimensional scans and limited generalization of deep learning networks when mapping random points to the target space [50]. This framework integrates a similarity learning (SL) network that maps sampled points into a representation space where valid points (those meeting target criteria) are grouped together while invalid points are pushed apart. This structured representation enhances scan convergence by refining how sampled points are organized and evaluated.
The system employs a dynamic sampling strategy that combines the SL network with trained VEGAS maps—adaptive importance sampling algorithms that progressively learn the distribution of promising parameters [50]. This integration mitigates initial convergence slowness when valid points are scarce and addresses suboptimal generalization that can occur due to network architecture limitations or poorly optimized hyperparameters.
Multi-Objective Optimization with Variable-Fidelity Machine Learning
For practical applications like antenna design or PQD optimization, researchers often face multiple competing objectives. A recent AI-based approach employs artificial neural network (ANN) surrogates combined with variable-fidelity electromagnetic (EM) simulations to efficiently identify Pareto-optimal solutions— designs representing the best possible trade-offs between conflicting goals [51]. This methodology generates multiple infill points in each iteration using a multi-objective evolutionary algorithm (MOEA) operating on the ANN surrogate, then refines the model with high-fidelity simulation data from these points [51].
The variable-fidelity approach is particularly valuable for computational efficiency. Initial sampling and ANN model construction occur at lower fidelity levels, with gradual increases to high-fidelity simulations as the algorithm progresses [51]. This strategy has demonstrated 40% speedup due to variable-fidelity modeling and nearly 90% savings compared to one-shot optimization approaches [51].
Experimental Protocols for AI-Guided PQD Synthesis
Data Collection and Preprocessing for ML Models
Implementing AI-guided parameter space mapping for PQD synthesis requires meticulous data collection and preprocessing. In a landmark study predicting properties of CsPbCl₃ PQDs, researchers compiled a comprehensive database from 59 peer-reviewed articles, extracting synthesis parameters and corresponding output properties [48]. The input features included:
- Injection temperature
- Chloride (Cl) source and amount (mmol)
- Lead (Pb) source and amount (mmol)
- Cesium (Cs) source and amount (mmol)
- Molar ratios (Cs:Pb, Cl:Pb)
- Amounts of octadecene (ODE), oleic acid (OA), and oleylamine (OLA) in milliliters
- Total ligand volume (OA+OLA) and ratios of Cl and Pb to ligand volume
The target output parameters were PQD size (nm), 1S absorption peak (nm), and photoluminescence (nm) [48]. The dataset comprised 708 data points (531 input, 177 output), which was deemed sufficient for accurate nanocrystal property prediction [48].
Table 2: Research Reagent Solutions for PQD Synthesis
| Research Reagent | Function in PQD Synthesis | Considerations for OA/OLA Ratio Studies |
|---|---|---|
| Oleic Acid (OA) | Carboxylic acid ligand; coordinates with surface atoms, controls growth direction [13] | Concentration affects binding to specific crystal facets; higher ratios promote specific morphologies [49] |
| Oleylamine (OLA) | Amine ligand; surface coordination, can act as reducing agent [13] | Binding strength differs from OA; ratio determines dominant surface termination [13] |
| OA/OLA Complex | Acid-base pair that may form in situ; acts as binary capping agent [13] | Ratio determines complex formation extent; influences reduction capacity and growth kinetics [13] |
| Cesium Precursors | Source of Cs ions for perovskite structure | Amount and molar ratios relative to Pb critical for stoichiometry [48] |
| Lead Precursors | Source of Pb ions for perovskite structure | Amount and type affect reactivity and incorporation [48] |
| Chloride Precursors | Halide component determining band structure | Amount and source influence optical properties [48] |
| Octadecene (ODE) | Non-polar solvent for high-temperature synthesis | Volume affects precursor concentrations and reaction kinetics [48] |
Data preprocessing followed a rigorous protocol including residual analysis to remove outliers (using z-score thresholding with ±3 standard deviations) and median imputation for missing values [48]. Feature engineering techniques included polynomial and logarithmic transformations to address skewness, while Principal Component Analysis (PCA) preserved approximately 95% of variance while enhancing computational efficiency [48]. The dataset was partitioned using hierarchical clustering into 80% training and 20% testing sets to prevent overfitting [48].
ML Model Training and Evaluation
The study employed six regression models: Support Vector Regression (SVR), Nearest Neighbour Distance (NND), Random Forest (RF), Gradient Boosting Machine (GBM), Decision Tree (DT), and Deep Learning (DL) [48]. All implementations used the scikit-learn library, with hyperparameter tuning performed via grid search. Model performance was evaluated using R² (coefficient of determination), Root Mean Squared Error (RMSE), and Mean Absolute Error (MAE) metrics [48].
While all models demonstrated high accuracy, SVR and NND achieved the best performance on both training and test datasets, with high R² values and low RMSE and MAE metrics [48]. This exceptional performance highlights ML's potential to accurately predict PQD properties based on synthesis parameters, including the critical OA/OLA ratio.
Case Study: AI-Guided Optimization of OA/OLA Ratio in PQD Synthesis
Impact of OA/OLA Ratio on Nanoparticle Morphology
The profound influence of the OA/OLA ratio on nanoparticle morphology has been systematically demonstrated in yttria (Y₂O₃) nanomaterials [49]. Experiments with varying ratios (100/0, 90/10, 65/35, and 50/50) of OLA to OA revealed distinct morphological transitions:
- 100/0 (OLA only): Produced regular and elongated hexagons
- 90/10: Resulted in lamellar pillars
- 65/35: Yielded irregular particles
- 50/50: Formed plates [49]
This morphological evolution was attributed to preferential adsorption of these ligands to different crystal facets. Oleylamine preferentially adsorbs onto {101} planes, while oleic acid preferentially binds to {111} planes [49]. As the OA proportion increases, it displaces OLA from specific surfaces, thereby altering growth kinetics and final morphology. This mechanistic understanding provides a foundation for ML models to predict morphological outcomes based on ligand ratios.
Flow Synthesis and AI Integration for Accelerated Development
Microscale flow synthesis technologies have emerged as powerful platforms for accelerated materials development, particularly when integrated with AI-guided decision-making strategies [39]. For lead halide PQDs, modular flow synthesis platforms equipped with precursor formulation, controlled flow synthesis, and in situ diagnostic modules enable high-throughput experimentation with real-time access to optoelectronic properties [39].
The convergence of these modular flow synthesis platforms with rapidly emerging artificial intelligence-guided decision-making strategies creates unprecedented opportunities for autonomous materials development [39]. This integrated approach is particularly valuable for optimizing multi-parameter systems like the OA/OLA ratio, where traditional one-variable-at-a-time approaches would be prohibitively time-consuming and resource-intensive.
Figure 1: AI-Guided Workflow for Parameter Space Mapping. This diagram illustrates the iterative process of ML-enhanced parameter optimization, incorporating adaptive sampling and variable-fidelity evaluations.
Future Directions and Implementation Guidelines
The integration of AI and ML into parameter space mapping represents a paradigm shift in nanomaterials research, particularly for optimizing complex parameter interactions like the OA/OLA ratio in PQD synthesis. Future advancements will likely focus on several key areas:
Autonomous Robotic Experimentation: Combining AI-guided decision-making with automated synthesis platforms will enable fully autonomous closed-loop optimization of PQD synthesis parameters [39].
Multi-Objective Optimization for Complex Applications: As applications for PQDs become more sophisticated, ML frameworks must evolve to handle multiple competing objectives simultaneously, similar to the variable-fidelity approaches used in antenna design [51].
Enhanced Generalization in High-Dimensional Spaces: Continued development of similarity learning networks and adaptive sampling techniques will improve convergence and generalization in increasingly complex parameter spaces [50].
For research teams implementing these strategies, we recommend starting with well-established ML models like SVR and RF, which have demonstrated excellent performance in PQD property prediction [48]. Implementation should include rigorous data collection protocols, appropriate feature engineering, and careful validation against experimental results. The OA/OLA ratio should be treated as a first-class parameter in these models, given its demonstrated significance in controlling nanoparticle morphology and properties [13] [49].
As AI-guided parameter space mapping continues to mature, it will undoubtedly accelerate the development of next-generation nanomaterials with tailored properties for specific applications, from photovoltaics and LEDs to medical imaging and quantum computing.
The journey of perovskite quantum dots (PQDs) from laboratory curiosities to commercially viable materials is primarily hindered by their susceptibility to degradation from environmental factors such as water, heat, and light. While compositional engineering and encapsulation strategies have been extensively explored, the strategic manipulation of surface ligand chemistry—specifically the ratio of oleic acid (OA) to oleylamine (ON)—has emerged as a powerful, fundamental approach to enhancing PQD stability. The oleic acid/oleylamine ligand pair represents a versatile combination in the synthesis of colloidal nanoparticles, serving not only as steric stabilizers to prevent aggregation but also as dynamic surface modulators that directly influence crystallization kinetics, facet-specific binding, and defect passivation [13]. This technical guide examines the critical impact of the OA:ON ratio on synthesizing PQDs with intrinsic resistance to degradation, framing this discussion within the broader context of advancing PQD technology toward practical application.
Fundamental Chemistry of the OA/ON Ligand System
The OA/ON ligand pair functions through a complex interplay of individual and synergistic effects that extend far beyond simple steric stabilization. Understanding their distinct chemical behaviors is prerequisite to effectively manipulating their ratio for stability enhancement.
Oleic Acid (OA): This carboxylic acid ligand (CH₃(CH₂)₇CH=CH(CH₂)₇COOH) binds to metal sites on the PQD surface through its carboxyl group, forming a relatively strong coordinate covalent bond. This binding is particularly effective on specific crystal facets, influencing morphological development during synthesis [13]. Its hydrophobic alkyl chain provides a primary barrier against water permeation.
Oleylamine (ON): This primary amine (C₁₈H₃₅NH₂) exhibits multifaceted roles in PQD synthesis. It coordinates to surface sites through its amine group, acts as a Lewis base for passivating undercoordinated lead atoms, and can even serve as a reducing agent [13] [52]. Commercially available oleylamine often contains impurities like the trans isomer (elaidylamine) which can affect its packing behavior and effectiveness, necessitating purification for reproducible research [52].
Synergistic Interactions: The combination of OA and ON can lead to the formation of an acid-base complex, an oleylammonium oleate ion pair, which may serve as a binary capping agent with distinct coordination and reduction capabilities compared to the individual ligands [13]. The different binding strengths and distinct binding modes of these two molecules on specific crystal facets directly affect reaction kinetics, ultimately directing the growth toward nanostructures with tailored characteristics [13].
Table 1: Fundamental Properties of the OA/ON Ligand System
| Property | Oleic Acid (OA) | Oleylamine (ON) |
|---|---|---|
| Chemical Class | Unsaturated carboxylic acid | Unsaturated primary amine |
| Molecular Formula | C₁₈H₃₄O₂ | C₁₈H₃₇N [52] |
| Binding Group | Carboxylate (-COO⁻) | Amine (-NH₂) |
| Primary Function | Steric stabilization, facet-specific binding | Coordination, reduction, defect passivation |
| Synergistic Effect | Forms acid-base complex with tunable surface binding | Forms acid-base complex with modified reduction potential |
Impact of OA:ON Ratio on PQD Synthesis and Properties
The ratio of OA to ON is a critical synthetic parameter that governs nucleation kinetics, growth rates, and final surface chemistry. Systematic variation of this ratio allows for precise control over structural and optical properties directly linked to PQD stability.
Morphological Control and Facet Stabilization
The OA:ON ratio exerts profound influence on PQD morphology through preferential adsorption onto specific crystal facets. A study on yttria nanomaterials, while not perovskite, provides a directly transferable principle: pure oleylamine (100/0 ON/OA ratio) produced regular and elongated hexagons, while the introduction of OA progressively transformed the morphology through lamellar pillars (90/10) and irregular particles (65/35) to plates (50/50) [49]. This morphological evolution was attributed to the preferential adsorption of ON onto {101} planes, while OA preferentially coated the {111} planes, effectively replacing ON from the surface and altering the relative growth rates of different crystal facets [49]. In PQDs, similar facet-specific binding can stabilize crystallographic planes that are less susceptible to ion migration or water ingress, thereby enhancing intrinsic stability.
Surface Chemistry and Defect Passivation
The balance between proton-donating (OA) and proton-accepting (ON) ligands at the PQD surface determines the density and nature of surface defects, which act as initiation sites for degradation.
Lead-Rich Surfaces: Surfaces with undercoordinated lead atoms are effectively passivated by the electron-donating amine groups of ON, reducing surface trap states that facilitate non-radiative recombination and photocatalytic degradation [13].
Halide-Rich Surfaces: Unpassivated halide sites can be stabilized through interaction with the ammonium ion of the oleylammonium oleate complex, reducing halide mobility and preventing vacancy formation [13].
Stoichiometric Balance: An optimal OA:ON ratio ensures a near-complete passivation of both cationic (Pb²⁺) and anionic (I⁻, Br⁻) surface sites, creating a robust, low-defect surface that is less chemically reactive and more resistant to attack from water and heat.
Table 2: Effect of OA:ON Ratio on PQD Characteristics and Stability
| OA:ON Ratio | Resulting PQD Characteristics | Implications for Stability |
|---|---|---|
| ON-rich (e.g., 100:0) | Anisotropic growth, elongated morphologies [49] | Possible enhanced charge transport but potentially higher surface energy facets |
| Balanced (e.g., 50:50) | Plate-like morphologies, mixed facet exposure [49] | Good overall passivation, balanced growth kinetics |
| OA-rich (e.g., 0:100) | Different morphological outcomes, potentially spherical | Possible incomplete cation site passivation, higher defect density |
| Intermediate (e.g., 65:35) | Irregular particles, complex facet structure [49] | May create defects at facet junctions but could slow ion migration |
Figure 1: Logical relationship between OA:ON ligand ratio manipulation and the resulting stability outcomes in perovskite quantum dots, showing how different ratios influence specific PQD properties.
Experimental Protocols for Ligand Ratio Optimization
Synthesis Methodology: Systematic Ratio Variation
A standardized approach to investigating the OA:ON parameter space is essential for obtaining reproducible, comparable results. The following protocol outlines a hot-injection method tailored for ratio optimization studies.
Precursor Preparation:
- Cationic Precursor: Dissolve lead bromide (PbBr₂, 0.2 mmol) in a mixture of 1-octadecene (5 mL) with varying OA:ON ratios.
- Ratios to Test: Prepare samples with ON:OA ratios of 100:0, 90:10, 65:35, and 50:50 to mirror the systematic approach demonstrated in morphology studies [49].
- Anionic Precursor: Dissolve cesium oleate (0.4 mmol) in 1-octadecene (3 mL) separately.
- Degassing: Dehydrate and degas both solutions under vacuum at 100°C for 60 minutes to remove residual water and oxygen.
Quantum Dot Synthesis:
- Under inert atmosphere (N₂ or Ar), heat the PbBr₂/OA/ON mixture to 150°C with vigorous stirring until a clear solution is obtained.
- Rapidly inject the preheated cesium oleate solution (100°C).
- Allow the reaction to proceed for 5-60 seconds (monitoring color development) before immediate cooling in an ice-water bath.
- Purify the resulting PQDs by centrifugation with anti-solvents (e.g., methyl acetate) and redisperse in anhydrous toluene.
Stability Assessment Using ISOS Protocols
To quantitatively evaluate the enhancement in stability conferred by optimal ligand ratios, standardized testing protocols are indispensable. The International Summit on Organic Solar Cells Stability (ISOS) protocols provide a modular, research-driven framework adapted for perovskite technologies [53].
Table 3: ISOS Stability Testing Protocols for PQD Assessment [53]
| Protocol | Stress Factors | Purpose | Key Parameters |
|---|---|---|---|
| ISOS-D (Dark Storage) | Oxygen, moisture, atmospheric components [53] | Tests intrinsic chemical stability & shelf life | T = 65°C/85°C; RH = 85% (ISOS-D-3) [53] |
| ISOS-L (Light Soaking) | Continuous illumination (1-sun equivalent) [53] | Accelerates photo-induced degradation | Light source spectrum, T = ambient/controlled |
| ISOS-T (Thermal Cycling) | Temperature cycles (e.g., -40°C to 85°C) [53] | Investigates stability to thermal expansion mismatch | Number of cycles, ramp rate, dwell time |
| ISOS-LC (Light-Dark Cycling) | Cyclic illumination (e.g., 6h light/6h dark) [53] | Reveals reversible "fatigue" effects & metastabilities | Cycle duration, light intensity |
| ISOS-I (Intrinsic Stability) | Any protocol in inert atmosphere [53] | Isolates intrinsic vs. extrinsic degradation | N₂ or Ar atmosphere, no encapsulation |
Implementation Guidelines:
- Sample Configuration: Test each OA:ON ratio variant in parallel using identical substrate, encapsulation (if any), and device architecture.
- Control Group: Include a reference sample with standard ligand ratio for comparative analysis.
- Environmental Control: For ISOS-D-3, use a controlled environmental chamber maintaining 85°C and 85% relative humidity [53].
- Data Collection: Monitor performance metrics (PLQY, absorbance, PCE for devices) periodically. Use T80 (time to 80% initial efficiency) or η₁₀₀₀ (efficiency after 1000 hours) as key figures of merit [53].
- Preconditioning: Document and standardize preconditioning (e.g., light soaking before testing) as cell history affects degradation kinetics [53].
Figure 2: Experimental workflow for systematically evaluating the impact of OA:ON ligand ratios on PQD stability using standardized ISOS testing protocols.
The Scientist's Toolkit: Essential Research Reagents
Successful investigation of ligand ratio effects requires carefully selected materials and characterization tools. The following table details essential components for such a study.
Table 4: Essential Research Reagents for PQD Ligand Ratio Studies
| Reagent/Material | Specifications | Primary Function |
|---|---|---|
| Oleic Acid (OA) | Technical grade, ≥90%; Purify via distillation if necessary | Carboxylic acid ligand for surface binding & stabilization [13] |
| Oleylamine (ON) | Technical grade, ≥70%; Purify to remove amides, nitroalkanes [52] | Amine ligand for coordination, reduction, defect passivation [13] [52] |
| Lead Bromide (PbBr₂) | Anhydrous, 99.99% trace metals basis | Pb²⁺ precursor for perovskite matrix |
| Cesium Carbonate (Cs₂CO₃) | 99.9% trace metals basis | Cs⁺ precursor for cesium oleate synthesis |
| 1-Octadecene (ODE) | Technical grade, 90%; Degas and purify before use | Non-coordinating solvent for high-temperature synthesis |
| Methyl Acetate | Anhydrous, 99.5% | Anti-solvent for PQD purification and precipitation |
| Toluene | Anhydrous, 99.8%, stored over molecular sieves | Dispersion solvent for purified PQDs |
The strategic manipulation of the oleic acid to oleylamine ratio represents a sophisticated approach to enhancing the intrinsic stability of perovskite quantum dots. By systematically varying this ratio, researchers can control facet-specific binding, improve surface passivation, and ultimately strengthen PQD resistance to water, heat, and light-induced degradation. When coupled with standardized ISOS stability assessment protocols, this approach provides a robust methodology for developing PQDs that maintain their exceptional optoelectronic properties under realistic operating conditions. Future research directions should focus on establishing quantitative structure-activity relationships between specific ligand ratios and degradation kinetics, exploring ternary ligand systems that incorporate additional functional molecules, and developing in-situ characterization techniques to observe ligand dynamics during stress testing. Through continued refinement of surface chemistry protocols, the path toward commercially viable, stable perovskite quantum dot technologies becomes increasingly attainable.
Benchmarking Performance: Validating OA/OAm-Optimized PQDs Against Alternative Materials and Methods
The optimization of the oleic acid (OA) and oleylamine (OAm) ratio is a critical parameter in the synthesis of perovskite quantum dots (PQDs), directly governing their optical performance by controlling surface chemistry and crystal growth. This whitepaper details how precise manipulation of this ligand ratio enables the achievement of high photoluminescence quantum yield (PLQY) and narrow full width at half maximum (FWHM). We elucidate the underlying mechanisms—including enhanced surface passivation and suppressed defect-mediated non-radiative recombination—through quantitative data, detailed experimental protocols, and mechanistic diagrams. This guide provides researchers and scientists with the foundational knowledge and practical methodologies to synthesize high-performance PQDs for advanced optoelectronic applications.
Perovskite quantum dots (PQDs), notably cesium lead halide (CsPbX₃) variants, have emerged as a transformative class of semiconducting nanomaterials due to their exceptional optoelectronic properties. These include wide excitation wavelength ranges, easily tunable emission wavelengths, and high color purity [38]. Two of the most critical performance metrics for display and lighting applications are high Photoluminescence Quantum Yield (PLQY) and narrow Full Width at Half Maximum (FWHM). PLQY measures the efficiency of photon conversion, with near-unity values being ideal for bright emission. Narrow FWHM, typically below 30 nm for green-emitting CsPbBr₃, ensures high color saturation and a wide color gamut [38] [54].
The pursuit of these superior metrics is intrinsically linked to the synthetic process, specifically the dynamic and complex surface chemistry governed by ligand interactions. The hot-injection method, a cornerstone technique for high-quality PQD synthesis, relies heavily on the presence and ratio of two key ligands: oleic acid (OA) and oleylamine (OAm) [38] [25]. These ligands do not merely act as stabilizers to prevent aggregation; they directly participate in the nucleation and growth stages, passivate surface defects, and determine the final optical properties of the PQDs. The balance between these ligands is therefore not a mere procedural detail but a fundamental variable that impacts the entire electronic structure of the resulting nanomaterial. This whitepaper dissects the mechanism by which the OA/OAm ratio optimizes performance, framing it within the broader research context of achieving precise synthetic control over PQD architecture.
Mechanistic Insights: How OA/OAm Optimization Enhances Performance
The synergistic interaction between OA and OAm on the PQD surface is the key to unlocking high performance. Their ratio directly influences two primary factors: surface passivation and crystal growth kinetics.
Surface Passivation and Defect Tolerance
The surface of a nascent PQD is rich in ionic sites that, if unpassivated, act as traps for charge carriers. These trap states facilitate non-radiative recombination, where energy is lost as heat instead of being emitted as light, thereby reducing the PLQY.
- The Ligand Binding Motif: On the CsPbBr₃ QD surface, OA and OAm do not exist as neutral molecules but as ion pairs. OAm selectively binds as oleylammonium bromide (OAm⁺-Br⁻), while OA binds as oleate (OA⁻) in the presence of OAm, forming oleylammonium oleate [25]. This creates a passivating layer that neutralizes charged surface defects.
- Dynamic Binding and Density: The binding of these ligands is highly dynamic. Nuclear Magnetic Resonance (NMR) studies quantify individual surface densities of OA and OAm at approximately 1.2–1.7 nm⁻² [25]. An optimized OA/OAm ratio ensures a near-complete monolayer coverage, effectively passivating the majority of surface sites and minimizing non-radiative pathways.
- Impact of Ratio Imbalance: An excess of OAm can lead to over-protonation of the surface, potentially creating new defects, while an excess of OA can result in poor colloidal stability and ligand desorption. The correct balance maximizes the number of passivated sites, directly leading to a higher PLQY.
Control of Crystal Growth and Size Distribution
The narrow FWHM of an emission spectrum is a direct consequence of a monodisperse population of QDs, where all particles are nearly identical in size and composition. The OA/OAm ratio is a powerful tool to achieve this monodispersity.
- Modulating Reactivity: The ligands control the availability of precursors and the kinetics of crystal growth. They adsorb onto the surface of growing nanocrystals, temporarily halting growth and allowing for focused size distribution through Ostwald ripening.
- Short-Chain vs. Long-Chain Ligands: Research demonstrates that using a shorter-chain amine like octylamine (OcAm) instead of OAm can yield MAPbBr₃ PQDs with higher size uniformity and higher PLQY, as shorter chains may allow for denser and more stable packing on the QD surface [15]. This highlights that the steric properties dictated by the ligand chain length, which are part of the ratio optimization, are crucial for monodispersity.
- Role in Low-Temperature Synthesis: In low-temperature syntheses, the optimized combination of OcAm and OA was shown to produce monodisperse PQDs without the co-formation of large particles, which is a common problem when using OAm [15]. This precise control over nucleation and growth directly results in a narrow FWHM.
The following diagram illustrates the synergistic workflow and logical relationship between ligand ratio and the final PQD performance metrics.
Quantitative Data: Performance Metrics of Optimized PQDs
The theoretical mechanisms are substantiated by quantitative experimental data. The following tables summarize key performance metrics achievable with optimized ligand conditions and the impact of different ligand types.
Table 1: Optical Performance of Optimized PQDs
| Perovskite Composition | Synthesis Method | Key Ligand Ratio / Type | PLQY (%) | FWHM (nm) | Reference |
|---|---|---|---|---|---|
| CsPbBr₃ | Hot-injection | Optimized OA/OAm | Up to 95% | ~19-25 | [38] [54] |
| MAPbBr₃ | Low-temperature | OcAm/OA (vs. OAm/OA) | Significantly Higher | Small Particle Size | [15] |
| CsPbBr₃ | Hot-injection | - | 50-90% | 12-40 | [55] |
Table 2: Impact of Ligand Properties on PQD Characteristics
| Ligand / Treatment | Function | Thermodynamic / Kinetic Effect | Result on PQDs |
|---|---|---|---|
| Oleic Acid (OA) | Acidic Ligand | Binds as oleate; dynamic equilibrium | Passivates lead-rich sites; controls growth |
| Oleylamine (OAm) | Amine Ligand | Binds as oleylammonium bromide | Passivates halide-rich sites; affects nucleation |
| 10-undecenoic acid | Carboxylic Acid | Exergonic exchange with oleate (Keq = 1.97) | Can improve PL intensity [25] |
| undec-10-en-1-amine | Amine | Exergonic exchange with OAm (Keq = 2.52) | Can improve PL intensity [25] |
| Octylamine (OcAm) | Short-chain Amine | Improved packing & stability vs. OAm | Higher monodispersity & PLQY [15] |
Experimental Protocols: Methodology for OA/OAm Optimization
To achieve the reported performance metrics, reproducible and precise experimental protocols are essential. Below is a detailed methodology for a standard hot-injection synthesis and a low-temperature ligand optimization.
Standard Hot-Injection Synthesis of CsPbBr₃ PQDs
This protocol is adapted from the seminal work by Protesescu et al., with modifications to study ligand thermodynamics [25].
Research Reagent Solutions:
| Reagent | Function | Role in Synthesis |
|---|---|---|
| Cesium Carbonate (Cs₂CO₃) | Cesium precursor | Provides Cs⁺ ions for the ABX₃ structure. |
| Lead Bromide (PbBr₂) | Lead & halide precursor | Source of Pb²⁺ and Br⁻ ions. |
| 1-Octadecene (ODE) | Non-coordinating solvent | High-booint solvent for the reaction medium. |
| Oleic Acid (OA) | Acidic ligand | Passivates surface; controls growth kinetics. |
| Oleylamine (OAm) | Amine ligand | Passivates surface; aids precursor solubility. |
| Toluene | Antisolvent | Used for purification and precipitation of PQDs. |
Step-by-Step Procedure:
- Cs-oleate Precursor: Load 0.4 g of Cs₂CO₃, 1.25 mL of OA, and 15 mL of ODE into a 50 mL 3-neck flask. Dry and degas under vacuum for 1 hour at 120°C. Then, heat under N₂ atmosphere to 150°C until all Cs₂CO₃ has reacted, forming a clear solution. Maintain at 100°C for later use.
- PbBr₂ Precursor Solution: In a separate 100 mL 3-neck flask, load 0.138 g of PbBr₂, 1.0 mL of OA, 1.0 mL of OAm, and 10 mL of ODE. This is the critical step for ligand ratio optimization. Systematic variation (e.g., OA:OAm from 1:2 to 2:1) should be performed in separate batches. Dry and degas the mixture under vacuum for 1 hour at 120°C.
- Hot-Injection and Reaction: Under N₂ flow, rapidly raise the temperature of the PbBr₂ solution to 160°C. Swiftly inject 1.0 mL of the preheated Cs-oleate precursor. The reaction proceeds for 5-10 seconds before being quenched by immersing the flask in an ice-water bath.
- Purification: Transfer the crude solution to centrifuge tubes. Add an antisolvent (e.g., toluene or ethyl acetate) and centrifuge at high speed (e.g., 14,000 rpm for 10 minutes) [15]. Discard the supernatant and re-disperse the pellet in a non-polar solvent like hexane or toluene. Repeat centrifugation at a lower speed (e.g., 6,000 rpm) to remove any aggregates. The final supernatant contains the purified PQDs.
Ligand Exchange and Thermodynamic Measurement
To quantitatively understand the ligand binding, an exchange experiment with diagnostic ligands can be performed, as detailed in [25].
- PQD Preparation: Synthesize CsPbBr₃ QDs using dodecylamine instead of OAm to avoid NMR spectral overlap.
- Titration and NMR Tracking: Titrate a ligand with a distinct NMR signal (e.g., 10-undecenoic acid) into a suspension of purified PQDs in toluene-d⁸.
- Quantification: Use ¹H NMR spectroscopy to track the concentrations of free and bound fractions of both the native and incoming ligands.
- Equilibrium Constant Calculation: The equilibrium constant (Keq) for the ligand exchange is calculated from these concentrations, providing a thermodynamic measure of binding strength.
The following diagram maps this experimental workflow and its key decision points.
The meticulous optimization of the oleic acid and oleylamine ratio is a quintessential example of how mastering fundamental surface chemistry can directly dictate the top-tier performance of advanced nanomaterials. By achieving a balanced ligand ratio, researchers can ensure superior surface passivation that suppresses non-radiative recombination (high PLQY) and precise control over crystal growth that ensures monodispersity (narrow FWHM). The quantitative data and protocols provided herein offer a roadmap for this optimization.
Future research in this domain is likely to focus on several key areas:
- Lead-Free PQDs: Exploring optimized ligand systems for non-toxic alternatives like Cs₃Bi₂X₉ and CsSnX₃, which have different surface chemistries [55].
- Advanced Ligands: Investigating stronger-binding ligands, such as phosphonic acids, which can undergo more irreversible exchange and further enhance stability and PL [25].
- Scalable Synthesis: Translating the insights from small-batch, hot-injection methods to scalable, low-temperature, and continuous-flow syntheses for industrial application [15] [12].
The pursuit of the perfect PQD continues to be guided by a deep understanding of the dynamic interactions at its surface, with the OA/OAm ratio remaining a central and powerful variable in the researcher's toolkit.
The application of luminescent nanomaterials in biomedicine has revolutionized areas such as bioimaging, biosensing, and drug delivery. Among these materials, perovskite quantum dots (PQDs), carbon dots (CDs), and cadmium telluride quantum dots (CdTe QDs) have emerged as particularly promising candidates due to their exceptional optical properties and nanoscale dimensions. This whitepaper provides a comprehensive technical comparison of these three nanomaterial classes, with particular emphasis on how the oleic acid (OA) to oleylamine (OAm) ratio in PQD synthesis influences their ultimate performance in biomedical contexts. Understanding these structure-property relationships is crucial for selecting appropriate materials for specific biomedical applications and for guiding the rational design of next-generation nanoprobes.
Fundamental Properties of Luminescent Nanomaterials
The distinctive physical and optical characteristics of PQDs, CDs, and CdTe QDs fundamentally dictate their suitability for various biomedical applications.
Table 1: Fundamental Properties of Luminescent Nanomaterials
| Property | Perovskite QDs (PQDs) | Carbon Dots (CDs) | CdTe QDs |
|---|---|---|---|
| Core Composition | CsPbBr₃, CH₃NH₃PbBr₃ [25] [26] | Carbon, nitrogen, oxygen [56] [57] | Cadmium telluride [58] [59] |
| Typical Size Range | 2-10 nm [26] | 2-10 nm [56] | 2-8 nm (size-dependent emission) [58] |
| Quantum Yield (QY) | Up to 100% [26] | Up to 83% [56] | 15% to 90% [58] |
| Emission Tunability | Excellent (via size & composition) [26] | Good (via precursor & doping) [56] | Excellent (via size) [58] |
| Biocompatibility | Moderate (Pb content concern) [60] | Excellent [60] [57] | Poor (Cd toxicity concerns) [60] |
Perovskite Quantum Dots (PQDs)
PQDs, particularly lead halide perovskites (CsPbX₃, CH₃NH₃PbX₃), are known for their narrow emission bands, high photoluminescence quantum yields (PL QY), and exceptionally easy bandgap tunability through both size control and halide composition [25] [26]. Their ionic crystal structure and low formation energy, however, make them susceptible to degradation in polar environments, a significant challenge for biological applications [25].
Carbon Dots (CDs)
CDs are biocompatible, fluorescent nanoparticles primarily composed of carbon, often doped with heteroatoms like nitrogen or sulfur [56] [57]. They are celebrated for their "green" synthesis from sustainable precursors, low toxicity, and tunable surface chemistry, which allows for easy functionalization with targeting moieties [61] [60] [56]. Their photoluminescence, while highly stable, often features broader emission spectra compared to PQDs and CdTe QDs.
Cadmium Telluride QDs (CdTe QDs)
CdTe QDs represent a classic heavy-metal-based QD system with well-established synthetic protocols for producing high-quality nanocrystals with size-tunable photoluminescence across the visible and near-infrared spectrum [58] [59]. Their primary drawback for biomedical use is the potential cytotoxicity of cadmium ions, which can leak upon surface degradation [60].
The Critical Role of OA:OAm Ratio in PQD Synthesis
The synthesis of high-quality PQDs is profoundly influenced by the ratio and dynamic binding of surface ligands, specifically oleic acid (OA) and oleylamine (OAm). These ligands are not merely passive stabilizers but play active, complex roles in nucleation and growth.
Ligand Functions and Binding Dynamics
Oleic Acid (OA) typically binds to the QD surface in its deprotonated form (oleate), coordinating with metal sites (e.g., Pb²⁺). Oleylamine (OAm) often binds as oleylammonium ions, interacting with halide anions on the surface [25]. Nuclear magnetic resonance (NMR) studies reveal that these ligands interact dynamically with the CsPbBr₃ QD surface, with individual surface densities of 1.2–1.7 nm⁻² [25]. The binding is highly influenced by the ionic nature of the perovskite lattice, and polar solvents can promote ligand desorption, leading to a loss of colloidal stability and PL QY [25].
Impact of OA:OAm Ratio on PQD Properties
The concentration ratio of OA to OAm is a critical synthetic parameter. Research on CH₃NH₃PbBr₃ NCs shows that increasing the total ligand concentration (OA and OAm) while keeping precursor concentration constant results in a blue-shift of the PL emission maximum. This indicates the formation of smaller nanocrystals due to a higher degree of quantum confinement, as a denser ligand shell suppresses crystal growth [26]. Furthermore, the PL intensity can be modulated by ligand exchange with stronger-binding species. For instance, the exergonic exchange of bound oleate with 10-undecenoate (Keq = 1.97) or of oleylamine with undec-10-en-1-amine (Keq = 2.52) increases the photoluminescence intensity of CsPbBr₃ QDs, correlating with improved surface passivation [25].
Detailed Experimental Protocols
Reproducible synthesis is foundational for reliable research and application. Below are detailed protocols for synthesizing each nanomaterial.
Protocol: Ligand-Assisted Reprecipitation (LARP) of CH₃NH₃PbBr₃ PQDs
This room-temperature method allows for bandgap tunability by varying precursor and ligand concentrations [26].
- Step 1: Precursor Solution Preparation. Dissolve variable amounts of PbBr₂ and CH₃NH₃Br in 0.5 mL of anhydrous N,N-Dimethylformamide (DMF). For a "standard" 1x concentration, use 0.02 mmol PbBr₂ and 0.016 mmol CH₃NH₃Br.
- Step 2: Ligand Addition. Add a fixed volume of ligands to the precursor solution. A typical ratio is 5 μL of oleylamine (OLA) and 50 μL of oleic acid (OA). To investigate the OA:OAm ratio effect, systematically vary these volumes while keeping other parameters constant.
- Step 3: Reprecipitation. Under vigorous stirring, quickly inject the 0.5 mL DMF aliquot into 5 mL of toluene (the poor solvent). A color change to yellow-green indicates immediate nanocrystal formation.
- Step 4: Purification. Allow larger agglomerates to precipitate naturally or separate via centrifugation (e.g., 5000 rpm for 5 min). Collect the clear supernatant containing the dispersed PQDs for characterization.
Protocol: Microwave-Assisted Synthesis of Carbon Dots
This is a rapid, green method for producing nitrogen-doped CDs (N@CQDs) from apricot juice [62].
- Step 1: Precursor Preparation. Obtain juice from Prunus armeniaca (apricots) by pitting the fruit and processing it in a mixer.
- Step 2: Microwave Carbonization. Place 50 mL of the juice in a conical flask and irradiate in a 900 W microwave for 5 minutes. The solution will turn brown.
- Step 3: Purification. Filter the resulting solution, sonicate for 20 minutes, and centrifuge at 4000 rpm for 10 minutes. Perform a final filtration through a 0.45 μm cellulose membrane. The final solution is stable and ready for use and characterization, with a reported quantum yield of up to 37.1% [62].
Protocol: Aqueous Synthesis of CdTe QDs Using Ammonia
This hydride-free, cost-effective method produces high-quality, water-soluble CdTe QDs [59].
- Step 1: Stock Solution Preparation. In a flask, combine 0.9 mmol of Cd salt (e.g., CdCl₂), 3.5 mmol of the thiol ligand (e.g., 3-mercaptopropionic acid, MPA), 8 mL of concentrated NH₃ solution (35%), and 0.2 mmol of Na₂TeO₃. Dilute the mixture to 130 mL with deionized water.
- Step 2: Inert Atmosphere. Sparge the solution with N₂ gas for 10-15 minutes to remove dissolved oxygen, which can oxidize tellurite precursors.
- Step 3: QD Growth. Reflux the solution under N₂ atmosphere. Monitor the growth by taking aliquots at different time intervals. The emission color tunability from green to red is achieved by controlling the refluxing time, which allows the nanocrystals to grow via an Ostwald ripening mechanism [59].
Quantitative Comparison for Biomedical Applications
A side-by-side comparison of key performance metrics is essential for material selection.
Table 2: Performance Metrics in Biomedical Applications
| Metric | Perovskite QDs (PQDs) | Carbon Dots (CDs) | CdTe QDs |
|---|---|---|---|
| Photostability | Moderate (ionic nature) [25] | Excellent [56] | High (with shelling) [60] |
| Aqueous Stability | Low (requires coating) [25] | Excellent [56] | Good (with hydrophilic ligands) [59] |
| In Vitro Toxicity | High (Pb leakage) [60] | Low (even at 400 µg/mL) [60] | High (Cd leakage) [60] |
| Bioimaging Performance | Bright but limited by stability & toxicity [60] | Biocompatible, good for long-term imaging [57] | Bright but cytotoxic concerns limit use [60] |
| Biosensing Performance | Promising, but environment-sensitive [61] | Excellent (e.g., Lisinopril LOD: 2.2 ng/mL) [62] | Excellent (e.g., pesticide detection) [58] |
The Scientist's Toolkit: Essential Research Reagents
Successful experimentation in this field requires a careful selection of specialized reagents.
Table 3: Essential Research Reagents for Nanomaterial Synthesis
| Reagent | Function | Example Use Case |
|---|---|---|
| Oleic Acid (OA) & Oleylamine (OAm) | Dynamic surface ligands for PQDs; control nucleation, growth, and stability [25] [26]. | LARP synthesis of CH₃NH₃PbBr₃ PQDs [26]. |
| 3-Mercaptopropionic Acid (MPA) | Thiol-based capping ligand; provides water solubility and surface passivation. | Aqueous synthesis of CdTe QDs [59]. |
| Ammonia Solution (NH₄OH) | Acts as a pH adjuster and reducing agent in "green" QD synthesis. | Reducing sodium tellurite in hydride-free CdTe QD synthesis [59]. |
| Carbohydrate Precursors (e.g., Glucose, Chitosan) | Renewable carbon sources for the "bottom-up" synthesis of CDs [56]. | Hydrothermal synthesis of carbohydrate-derived CDs (CDCQDs) [56]. |
| Nitrogen-Doping Agents (e.g., Urea, APTMS) | Enhance the quantum yield and modify the electronic structure of CDs [56]. | Creating N@CQDs with high quantum yield for biosensing [62]. |
This comparative analysis elucidates that there is no universally superior luminescent nanomaterial; rather, the optimal choice depends heavily on the specific biomedical application and its requirements for toxicity, stability, and optical performance. Carbon dots currently hold the greatest advantage in applications where biocompatibility and low toxicity are paramount, such as in vivo imaging and drug delivery. CdTe QDs, despite their toxicity concerns, remain a benchmark for high-performance optical applications where their brightness and tunability are critical and environmental release is controlled. Perovskite QDs offer exceptional optical properties and easy synthesis but are currently hampered by lead toxicity and instability in aqueous media, limiting their practical biomedical use.
Future research directions should focus on the development of heavy-metal-free PQDs and robust encapsulation strategies to enhance their stability. For all QD types, deeper investigation into the long-term fate in biological systems and thorough biodistribution studies are crucial for clinical translation. Furthermore, as demonstrated, the rational design of surface chemistry, exemplified by the precise control of the OA:OAm ratio in PQD synthesis, will continue to be a fundamental strategy for tailoring nanomaterial properties to meet the rigorous demands of modern biomedicine.
The pursuit of ultrasensitive detection of target molecules within complex biological media represents a frontier in analytical chemistry and biosensing. Success in this area is pivotal for advancing clinical diagnostics, where biomarkers often exist at trace concentrations amidst a background of interfering substances. This technical guide examines the core principles and methodologies for validating sensor performance, focusing on the detection of the neurotransmitter dopamine in human serum. The selection and synthesis of the sensing material are foundational to this process. The discussion is framed within a broader research context: investigating the impact of the oleic acid (OA) to oleylamine (OAm) ratio during the synthesis of perovskite quantum dots (PQDs), a class of nanomaterials with exceptional optoelectronic properties, and how this ratio ultimately influences the sensor's sensitivity, selectivity, and anti-fouling capabilities in complex media [63].
The Critical Role of Surface Chemistry in PQD Synthesis
The surface chemistry of nanomaterials, governed by their capping ligands, is a critical determinant of their performance in sensing applications. For PQDs, the ratio of OA to OAm used during synthesis is not merely a solubility aid but a powerful tool to control the nanocrystal's properties and its subsequent interaction with analytes and complex matrices.
Functions of OA and OAm Ligands
- Size and Crystallinity Control: The OA/OAm ratio exerts a profound influence on the nucleation and growth kinetics of PQDs. A higher proportion of OAm has been observed to induce a blue shift in the photoluminescence (PL) emission of CH₃NH₃PbBr₃ PQDs, indicating the formation of smaller-sized nanocrystals due to stronger quantum confinement [63]. This size control directly affects the bandgap and optical properties of the resulting PQDs.
- Surface Passivation and Stability: Ligands passivate the surface of PQDs, neutralizing charge defects that would otherwise act as non-radiative recombination centers, quenching photoluminescence. An optimal balance of OA and OAm is crucial for achieving high photoluminescence quantum yields (PLQY), which have been reported to reach as high as 93% for CH₃NH₃PbBr₃ PQDs [7]. Furthermore, a synergetic effect between an excess of these ligands and incorporation of silica (SiO₂) has been shown to dramatically enhance PL stability, with composites retaining 70% of initial intensity after 20 days in ambient conditions [63].
- Interfacial Interaction with Analytes: The ligand shell is the first point of contact between the PQD and the target molecule. A carefully tuned OA/OAm ratio can create a surface environment that favors specific interactions, such as π-π stacking or electrostatic attraction, with the analyte of interest. For instance, a specific OA/OAm ratio of 1:0.1 was selected for one study due to its high PL intensity, which is a prerequisite for sensitive fluorescence-based detection [63].
Experimental Protocols for Sensor Development and Validation
This section provides detailed methodologies for fabricating and rigorously evaluating PQD-based dopamine sensors, with particular attention to the role of synthesis conditions.
Synthesis of CH₃NH₃PbBr₃ PQDs with Controlled OA/OAm Ratio
The ligand-assisted reprecipitation (LARP) method is a common and effective technique [63] [7].
- Reagents: Lead(II) bromide (PbBr₂, ≥99.999%), methylammonium bromide (CH₃NH₃Br), oleylamine (OAm, 80-90%), oleic acid (OA, 90%), anhydrous N,N-Dimethylformamide (DMF), and toluene.
- Procedure:
- Co-dissolve PbBr₂ and CH₃NH₃Br in DMF within an inert atmosphere to prevent degradation.
- Add predetermined molar ratios of OA and OAm to the precursor solution. For example, ratios such as 1:1, 1:0.5, and 1:0.1 (OA:OAm) should be prepared to systematically study the ligand effect [63].
- Vigorously stir the mixture until a clear, transparent solution is obtained.
- Quickly inject a precise volume of this precursor solution into toluene that has been pre-heated or cooled to a set temperature (e.g., 0°C to 60°C) [7].
- Immediately observe the formation of a luminescent colloidal solution, indicating PQD formation.
- Purify the PQDs by centrifugation to remove large aggregates and unreacted precursors. Re-disperse the purified PQDs in a non-polar solvent like toluene for further use.
Fabrication of a Dual-Mode CsPbBr₃-PQD-COF Nanocomposite Sensor
A advanced sensing platform can be constructed by integrating PQDs into a covalent organic framework (COF) to enhance stability and selectivity [64].
- Reagents: CsPbBr₃ PQDs, 1,3,5-tris(4-aminophenyl)benzene (TAPB), 2,5-dihydroxyterephthalaldehyde (DHTA), rhodamine B, and anhydrous solvents.
- Procedure:
- Synthesize CsPbBr₃ PQDs: Use a hot-injection method. Dissolve CsBr and PbBr₂ in DMF with OA and OAm in an inert atmosphere. Heat to 120°C and rapidly inject preheated toluene to trigger nucleation. Quench the reaction after 10 seconds to obtain PQDs with high PLQY [64].
- Prepare COF Matrix: Synthesize the COF via Schiff-base condensation by reacting TAPB and DHTA in DMF, catalyzed by acetic acid, to form a highly ordered and porous π-conjugated scaffold [64].
- Integrate PQDs into COF: Incorporate the as-synthesized CsPbBr₃ PQDs into the COF precursor solution. The COF acts as a stable host, protecting the PQDs and facilitating analyte diffusion through its pores.
- Add Visual Indicator: Incorporate rhodamine B into the nanocomposite to provide a visual colorimetric readout, shifting from green-to-pink at dopamine concentrations above 100 pM [64].
Validation in Complex Media: Anti-Fouling and Selectivity Testing
A key validation step is assessing sensor performance in a complex, protein-rich environment like human serum or brain homogenate.
- Sample Preparation: Dilute human serum or spike dopamine-depleted brain homogenate with known concentrations of dopamine [64] [65].
- Interference Testing: Introduce common biological interferents, such as ascorbic acid (AA) and uric acid (UA), at concentrations significantly higher than dopamine (e.g., 100-1000 times) to challenge the sensor's selectivity [64].
- Recovery Assay: Measure the dopamine concentration in the spiked real-world samples using the calibrated sensor. Calculate the recovery percentage as (Measured Concentration / Spiked Concentration) × 100%. Excellent sensors demonstrate recovery rates close to 100% (e.g., 97.5–103.8% in human serum) [64].
- Long-Term Stability Test: Monitor the sensor's signal response over multiple days or weeks to ensure its robustness and operational lifetime.
The experimental workflow from synthesis to validation is illustrated below.
Performance Metrics and Comparative Analysis
Evaluating sensor performance requires a standardized set of quantitative metrics. The table below summarizes the performance of various advanced dopamine sensing platforms, highlighting the exceptional results achievable with nanomaterial-based approaches.
Table 1: Performance Comparison of Ultrasensitive Dopamine Sensors
| Sensing Platform | Detection Principle | Limit of Detection (LOD) | Linear Range | Performance in Complex Media | Key Advantage |
|---|---|---|---|---|---|
| CsPbBr₃-PQD-COF [64] | Fluorescence & Electrochemical Impedance | 0.3 fM (Fluorescence)2.5 fM (EIS) | 1 fM to 500 µM | Recovery: 97.5-103.8% (Serum); Excellent specificity | Dual-mode detection; Visual readout |
| Graphene Aptasensor [65] | Field-Effect Transistor | 1 aM | 1 aM to 100 µM | Effective in brain homogenate & CSF | Wide dynamic range; High scalability |
| Ti₃C₂Tₓ-MXene/4-APBA [66] | Electrochemical | 1.3 nM (Buffer)1.9 nM (Serum) | 40 to 500 nM | Specific detection in serum | High sensitivity (0.0134 µA/nM) |
| Mesoporous SiO₂/Au [67] | Electrochemical (DPV) | 0.084 µM | Not Specified | Demonstrated in human serum | Excellent anti-fouling & anti-interference |
| Nucleic Acid Aptamer-based [68] | Electrochemical | 1 aM (10⁻¹⁸ M) | Not Specified | Not Specified | Record-low LOD for electrochemical sensing |
The relationships between different sensing modalities and their performance characteristics can be visualized as follows.
The Scientist's Toolkit: Essential Research Reagents and Materials
The development and validation of high-performance sensors rely on a suite of specialized materials and reagents. The following table details the core components used in the featured experiments.
Table 2: Essential Research Reagent Solutions for PQD Dopamine Sensor Development
| Material/Reagent | Function in Experiment | Key Properties & Considerations |
|---|---|---|
| Lead Bromide (PbBr₂) | Precursor for perovskite crystal structure (B-site cation) [64] [7] | High purity (≥99.999%) is critical for optimal photoluminescence quantum yield and reduced defects. |
| Cesium Bromide (CsBr) / Methylammonium Bromide (CH₃NH₃Br) | Precursor for A-site cation in perovskite structure (ABX₃) [64] [7] | Determines crystal stability and tolerance factor. Cs⁺ offers enhanced thermal stability. |
| Oleic Acid (OA) & Oleylamine (OAm) | Capping ligands and surface passivants during PQD synthesis [63] | Ratio controls PQD size, stability, and PLQY. Dynamic binding requires optimization to prevent detachment. |
| Covalent Organic Framework (COF) | Porous scaffold for PQD integration [64] | Provides high surface area, stabilizes PQDs, enhances selectivity via molecular sieving and π-π interactions. |
| Ti₃C₂Tₓ-MXene | 2D conductive nanomaterial for electrode modification [66] | Excellent metallic conductivity, hydrophilic surface, and functional groups for biomolecule conjugation. |
| DNA Aptamer | Biorecognition element for selective dopamine binding [65] | Short DNA strand that binds target with high affinity and specificity, enabling ultra-low LOD. |
| Human Serum / Brain Homogenate | Complex biological media for validation [64] [65] | Contains proteins, salts, and interferents (AA, UA) essential for testing real-world applicability and anti-fouling. |
The successful validation of sensing performance in complex media is a multi-faceted challenge that hinges on the intelligent design and synthesis of the sensing material. As explored in this guide, the OA/OAm ratio in PQD synthesis is a profound example of a fundamental chemical parameter that ripples outward, influencing nanocrystal size, optical stability, and ultimately, the sensor's ability to perform reliably in a demanding environment like human serum. The impressive analytical figures of merit—such as detection limits in the femtomolar to attomolar range and excellent recovery rates in biological samples—achieved by modern sensors underscore a significant leap forward. These advancements, built upon a deep understanding of surface chemistry and material integration, pave the way for the next generation of diagnostic tools capable of precise, real-time monitoring of biomarkers in clinical and pharmaceutical settings.
The convergence of nanomaterial science and soft matter physics has catalyzed significant advancements in wearable sensor technology. Among the most promising developments are polymer quantum dots (PQDs), carbon-based nanoscale semiconductors known for their tunable photoluminescence and high quantum yield. A critical challenge in their application has been environmental instability, particularly under the variable mechanical, thermal, and hydration conditions experienced by wearable devices. This technical guide explores the enhanced stability and long-term performance of PQDs through their integration into hydrogel matrices, with a specific focus on how the oleic acid (OA) to oleylamine (OLAM) ratio during PQD synthesis dictates their final characteristics and compatibility with hydrogel networks. We frame this discussion within the broader thesis that precise ligand engineering during nanocrystal synthesis is a fundamental determinant of performance in composite material systems [69].
Hydrogels, three-dimensional networks of hydrophilic polymers, provide an ideal environment for PQDs in sensing applications. Their high water content facilitates biocompatibility and conformal contact with biological tissues, while their tunable mechanical properties allow for matching the modulus of human skin, thus enabling the development of comfortable, long-term wearable sensors [70] [71]. Furthermore, the porous structure of hydrogels permits the efficient diffusion of analytes, making them excellent substrates for chemical and biological sensing. The integration of PQDs into these matrices combines the optical and electronic superiority of PQDs with the biomechanical advantages of hydrogels, creating a synergistic composite material ideal for next-generation wearables [72].
The Critical Role of the OA/OLAM Ligand Pair in PQD Synthesis
The surface chemistry of PQDs, governed by the ligand shell, is paramount for determining their optical properties, colloidal stability, and ultimately, their performance within a composite hydrogel matrix. The OA/OLAM ligand pair has emerged as a versatile combination in the synthesis of colloidal nanoparticles, including PQDs [69]. These ligands belong to different molecular categories—OA is a carboxylic acid, while OLAM is a primary amine—and their distinct binding strengths and binding modes on specific crystal facets profoundly affect the reaction kinetics and resulting nanostructure.
Ligand Functions and Impact of Ratio Variation
The table below summarizes the core functions of each ligand and the anticipated impact of varying their ratio in the synthesis medium.
Table 1: Functions and Effects of the OA/OLAM Ligand Pair in PQD Synthesis
| Ligand | Primary Function | Impact of High Ratio | Impact of Low Ratio |
|---|---|---|---|
| Oleic Acid (OA) | - Coordination Ligand: Binds to Pb²⁺ sites on PQD surface [69].- Steric Hindrance: Provides colloidal stability via long carbon chain.- Surface Passivation: Reduces surface defects. | - Softer, less crystalline PQDs.- Broader emission spectra (FWHM).- Potential for reduced photoluminescence quantum yield (PLQY). | - Smaller PQD core size.- Increased non-radiative recombination.- Poor colloidal stability. |
| Oleylamine (OLAM) | - Nucleation Control: Modulates precursor reactivity [69].- Reducing Agent: Can facilitate precursor conversion.- Shape Director: Binds preferentially to specific crystal facets. | - Faster reaction kinetics.- Anisotropic growth (e.g., nanorods, sheets).- Potential for broader size distribution. | - Slower nucleation and growth.- More spherical, isotropic morphology.- Lower overall yield. |
| OA:OLAM Complex | - Synergistic Capping: Forms an acid-base complex that acts as a binary capping agent [69].- Fine-Tuned Surface Energy: Allows for precise control over growth rate and final morphology. | - Intermediate growth rate.- Highly uniform, spherical PQDs.- Optimal surface coverage and stability. | - Incomplete surface passivation.- Increased defect density.- Susceptibility to oxidation and degradation. |
Connecting Ligand Ratio to Hydrogel Integration
The OA/OLAM ratio is not merely a synthesis parameter; it is a critical determinant of PQD post-synthetic processability and integration potential. PQDs synthesized with an optimal OA/OLAM ratio exhibit a densely packed, stable ligand shell that minimizes surface defects—the primary sites for photo-oxidation and ion leakage [69]. This inherent stability is a prerequisite for surviving the aqueous, ionic environment of a hydrogel. Furthermore, the terminal vinyl groups (-CH=CH₂) on both OA and OLAM can be leveraged for covalent grafting onto polymer chains during hydrogel cross-linking, creating a robust, integrated network that prevents PQD leaching and enhances long-term performance [72].
Experimental Protocols for PQD-in-Hydrogel Composite Fabrication
Synthesis of PQDs with Controlled OA/OLAM Ratios
Objective: To synthesize green-emitting PQDs with high PLQY and stability, optimized for integration into a PVA-based hydrogel.
Materials:
- Lead bromide (PbBr₂, 99+%)
- Cs-oleate (0.1 M in octadecene)
- Oleic Acid (OA, 90%)
- Oleylamine (OLAM, 90%)
- Octadecene (ODE, 90%)
- Acetone, Ethanol (for purification)
Procedure:
- Ligand Stock Solution: Prepare a 25 mL three-neck flask with a mixture of ODE (10 mL), OA, and OLAM. The OA:OLAM volume ratio should be systematically varied (e.g., 1:3, 1:1, 3:1) across different batches to study its effect.
- Precursor Reaction: Add PbBr₂ (0.188 mmol) to the flask. Degas and dry under vacuum at 120 °C for 1 hour until the PbBr₂ is fully dissolved.
- Injection and Nucleation: Under a N₂ atmosphere, rapidly raise the temperature to 180 °C and inject 0.4 mL of Cs-oleate solution. Let the reaction proceed for 30 seconds.
- Quenching and Purification: Immediately cool the reaction flask in an ice-water bath. Purify the crude solution by centrifugation with acetone/ethanol, then redisperse the PQD pellet in hexane for characterization [69] [21].
Characterization: Analyze PQD size and morphology via TEM. Measure absorption and photoluminescence spectra to determine emission peak, FWHM, and Stokes shift. Calculate PLQY using an integrating sphere.
Fabrication of PQD-Embedded PVA-Based Hydrogel Electrolyte
Objective: To create a stable, conductive, and stretchable hydrogel composite incorporating the synthesized PQDs for use as a sensing element.
Materials:
- Polyvinyl Alcohol (PVA, Mw 89,000-98,000)
- Synthesized PQDs (from Protocol 3.1)
- Dimethyl sulfoxide (DMSO)
- LiCl or other salt (for ionic conductivity)
- Deionized Water
Procedure:
- PVA Solution Preparation: Dissolve PVA powder (1 g) in a mixture of deionized water (6 mL) and DMSO (2.3 mL) at 90 °C with constant stirring until a clear solution is obtained. The DMSO acts as an anti-freezing agent, broadening the operational temperature range of the final sensor [72].
- PQD Incorporation: Slowly add 2 mL of the purified PQD solution in hexane to the PVA solution under vigorous stirring. Allow the hexane to evaporate, leaving the PQDs uniformly dispersed in the polymer solution.
- Cross-linking and Shaping: Subject the PQD/PVA mixture to freeze-thaw cycling (e.g., -20 °C for 12 hours, then 25 °C for 2 hours) to induce physical cross-linking via crystalline domain formation in PVA [72] [70].
- Electrolyte Loading (Optional): For conductive sensor applications, equilibrate the formed hydrogel in a LiCl solution (e.g., 5 M) to introduce mobile ions. The PQDs, with their surface functional groups, can help regulate ion flux and reduce detrimental side reactions like dendrite formation in electrochemical sensors [72].
Characterization: Perform cyclic compression/tensile tests to evaluate mechanical properties. Use electrochemical impedance spectroscopy (EIS) to measure conductivity. Assess long-term PL stability under continuous illumination and cyclic strain.
Diagram: PQD-Hydrogel Composite Fabrication Workflow
The following diagram visualizes the integrated experimental workflow from PQD synthesis to functional sensor fabrication.
Quantitative Data on Stability and Performance
The success of the integration strategy is validated by quantifying the enhancement in key performance metrics. The data below, synthesized from recent literature, demonstrates the superior stability of PQDs within hydrogel matrices.
Table 2: Long-Term Performance Metrics of PQDs in Hydrogel vs. Solution
| Performance Metric | PQDs in Solution (Control) | PQDs in PVA Hydrogel Matrix | Testing Conditions |
|---|---|---|---|
| Photoluminescence Retention | ~40% after 7 days | >95% after 30 days [72] | Ambient conditions, continuous white light (0.5 mW/cm²) |
| Thermal Stability | ~80% PL loss | <5% PL loss [73] | 120°C for 5 cycles (1 hour/cycle) |
| Operational Cycling | Significant degradation after 100 cycles | >1000 stable cycles [72] [74] | 0–100% strain at –20 °C |
| Ionic Stability | Rapid quenching in high ionic strength | Stable emission [72] | Immersion in 5M LiCl solution for 24h |
| Mechanical Integrity | N/A | Stable conductivity under 100% strain [71] [75] | Tensile testing |
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 3: Key Reagents for PQD-Hydrogel Composite Research
| Reagent/Material | Function | Application Note |
|---|---|---|
| Oleic Acid (OA) & Oleylamine (OLAM) | Surface Ligands: Control PQD nucleation, growth, and final surface chemistry. The ratio is critical for stability [69]. | Use high-purity grades (>90%). Store under inert atmosphere to prevent oxidation. |
| Polyvinyl Alcohol (PVA) | Hydrogel Matrix: Provides a biocompatible, mechanically tunable, and water-retentive network [72] [70]. | Molecular weight (e.g., 89k-98k Da) and degree of hydrolysis affect mechanical strength and crystallinity. |
| Dimethyl Sulfoxide (DMSO) | Anti-freeze Co-solvent: Lowers the freezing point of the hydrogel, enabling low-temperature operation [72]. | Enhances ion transport and prevents hydrogel crystallization at sub-zero temperatures. |
| Lithium Chloride (LiCl) | Ionic Conductor: Imparts electrical conductivity to the hydrogel for electrophysiological sensing [72] [71]. | Concentration can be tuned to balance conductivity and hydrogel swelling/skin irritation. |
| Ammonium Persulfate (APS) | Oxidizing Agent: Used for the in-situ polymerization of conductive polymers like PEDOT within hydrogels [75]. | Enables the formation of electronically conductive hybrid hydrogels. |
This technical guide has established a clear experimental and theoretical framework for developing highly stable PQD-hydrogel composites for wearable sensors. The evidence confirms that the OA/OLAM ligand ratio during PQD synthesis is a foundational parameter that transcends mere crystal growth, directly influencing the nanomaterial's resilience and its successful integration into a soft, hydrated polymer network. The resulting composites demonstrate unprecedented long-term optical and mechanical stability under conditions relevant to real-world wearable applications, including cyclic strain, variable temperatures, and prolonged operation.
Future work in this field will likely focus on expanding the functional complexity of these composites. This includes developing multi-stimuli responsive systems (e.g., pH- and temperature-sensitive) for advanced diagnostics [76] [77], and integrating energy storage units like zinc-ion batteries directly into the sensor patch using the same hydrogel electrolyte [72]. Furthermore, the adoption of advanced manufacturing techniques like 3D bioprinting and electrohydrodynamic printing will enable the creation of complex, patterned sensor architectures with high spatial resolution, pushing the boundaries of what is possible in wearable health monitoring and soft robotics [74] [21]. The precise engineering of nanomaterial surfaces, as demonstrated by the critical role of the OA/OLAM pair, will remain a central theme in enabling these next-generation technologies.
The exceptional optoelectronic properties of perovskite quantum dots (PQDs), including high photoluminescence quantum yield (PLQY), narrow emission spectra, and tunable bandgaps, have generated significant interest for biomedical applications such as biosensing and bioimaging. [19] [78] However, the clinical translation of conventional lead-based PQDs faces substantial barriers due to the inherent toxicity of lead and the poor aqueous stability of these materials. [79] [80] Consequently, research has pivoted toward developing lead-free alternatives that mitigate toxicity concerns while maintaining performance. The synthesis of these PQDs frequently employs the oleic acid (OA) and oleylamine (OAm) ligand pair, a combination critical for controlling nanocrystal growth, passivating surface defects, and determining colloidal stability. [13] This technical guide provides a comprehensive assessment of lead-free PQDs synthesized with OA/OAm, evaluating their toxicity profiles, biocompatibility, and potential for clinical application within the broader context of understanding how the OA/OAm ratio impacts PQD synthesis research.
Lead-Free Perovskite Quantum Dots: Materials and Properties
Lead-free PQDs are engineered by substituting the toxic lead (Pb²⁺) with environmentally benign metal cations such as bismuth (Bi³⁺), copper (Cu²⁺), or tin (Sn²⁺). These materials exhibit distinct structural and optical characteristics compared to their lead-based counterparts.
- Bismuth-Based PQDs (e.g., Cs₃Bi₂Br₉): These PQDs feature a zero-dimensional (0D) crystal structure with isolated [Bi₂Br₉]³⁻ dimers, as opposed to the connected octahedra in 3D lead perovskites. This results in strong quantum confinement, broader emission spectra (FWHM ~40–60 nm), and typically lower PLQY. [19] [80] However, they offer significantly lower toxicity and enhanced stability.
- Copper-Based PQDs (e.g., CsCuCl₃): These materials represent another class of lead-free alternatives. When functionalized with biomolecules like glutathione (GSH), they can achieve good water dispersibility and exhibit bright blue fluorescence, making them suitable for sensing applications in aqueous environments. [79]
The OA/OAm ligand pair plays a multifaceted role in the synthesis of these materials, not only controlling size and morphology but also passivating surface defects, which is crucial for enhancing the PLQY and stability of lead-free variants that often suffer from high defect densities. [13] [80]
Table 1: Characteristics of Representative Lead-Free PQDs Synthesized with OA/OAm Ligands
| Perovskite Composition | Crystal Structure | Emission Peak (nm) | PLQY (%) | Reported Stability | Primary Clinical Application Prospect |
|---|---|---|---|---|---|
| Cs₃Bi₂Br₉ | 0D (Dimmer) | ~430 (Blue) | 21.2 - 53 [80] | >97% FL after 72h [80] | Biosensing, Bioimaging |
| GSH-CsCuCl₃ | N/A | 447 (Blue) | 19.65 [79] | Good water dispersibility [79] | Biomarker Sensing (Serotonin) |
Quantitative Toxicity and Performance Assessment
A critical step in evaluating clinical potential is the direct comparison of lead-free PQDs against traditional materials and other nanomaterials. The following table benchmarks key performance and toxicity-related metrics.
Table 2: Performance and Toxicity Benchmarking of PQDs and Alternative Nanomaterials
| Nanomaterial Type | Exemplary LOD | Exemplary Target | Aqueous Stability | Toxicity Profile | Key Advantages |
|---|---|---|---|---|---|
| Lead-Based PQDs (e.g., CsPbX₃) | 0.1 nM [19] | Heavy Metal Ions [19] | Low (Requires encapsulation) [19] [78] | High (Pb toxicity) [19] [80] | High PLQY (50-90%), Ultra-sensitive [19] |
| Lead-Free PQDs (e.g., Cs₃Bi₂Br₉) | N/A | Cu²⁺ Ions [81] | Moderate to High [80] [81] | Low (Green metal) [80] | Eco-friendly, Good stability [19] [80] |
| Carbon QDs | N/A | N/A | High [78] | Low (Biocompatible) [78] | Excellent biocompatibility, Low cost [78] |
Experimental Protocols for Synthesis and Biocompatibility Testing
Synthesis of Lead-Free Cs₃Bi₂Br₉ PQDs Using OA/OAm
This protocol is adapted from eco-friendly synthesis methods for bismuth-based PQDs. [80]
- Materials:
- Precursors: Cesium bromide (CsBr, 99.5%), Bismuth bromide (BiBr₃, ≥98%).
- Solvents: 1-Octadecene (ODE, 90%).
- Ligands: Oleic Acid (OA, 90%), Oleylamine (OAm, 80-90%).
- Equipment: Three-neck flask, Schlenk line, Heating mantle, Centrifuge.
- Procedure:
- Degassing: Combine PbI2 (0.94 mmol) and ODE (40 mL) in a 250 mL 3-neck flask. Degas under vacuum at 120 °C for 1 hour. [82]
- Ligand Injection: Under a nitrogen atmosphere, inject OA and OAm (in a typical volume ratio of 2:1) into the flask. [82]
- Precursor Injection: Cool the solution to 80 °C. Rapidly inject a preheated precursor solution containing formamidinium oleate.
- Reaction and Quenching: Allow the reaction to proceed for a few seconds before rapidly cooling the mixture using an ice-water bath.
- Purification: Add an anti-solvent (e.g., 2-pentanol or methyl acetate) to the crude solution and centrifuge at 8000 rpm for 5 minutes. Precipitate the PQDs and disperse them in a non-polar solvent like hexane for storage. [82]
- Purification and Ligand Exchange: The purification process is critical for removing excess ligands and precursors. For enhanced water stability, a ligand exchange or surface functionalization step can be introduced using molecules like glutathione (GSH), as demonstrated for CsCuCl₃ PQDs. [79]
Methodology for Cytotoxicity and Biocompatibility Assessment
Robust assessment is mandatory for evaluating clinical potential. The following workflow outlines key experiments, though specific in-vivo toxicity data for OA/OAm-capped lead-free PQDs is limited in the provided search results.
Diagram 1: Biocompatibility assessment workflow for lead-free PQDs.
The Scientist's Toolkit: Essential Research Reagents
This table catalogs key reagents and materials essential for working with OA/OAm-synthesized lead-free PQDs in a research setting.
Table 3: Key Research Reagent Solutions for Lead-Free PQD Development
| Reagent / Material | Function in R&D | Example Specification / Note |
|---|---|---|
| Oleic Acid (OA) | Surface ligand, co-surfactant; controls crystal growth, passivates surface defects. [13] | Technical grade (90%); requires purification for reproducible synthesis. |
| Oleylamine (OAm) | Surface ligand, co-surfactant, reducing agent; critical for size and morphology control. [13] | Technical grade (80-90%); ratio with OA fine-tunes kinetic growth. |
| Cesium Bromide (CsBr) | 'A-site' precursor for all-inorganic perovskite (e.g., Cs₃Bi₂Br₉). [80] | High purity (≥99.5%) recommended for optimal optical properties. |
| Bismuth Bromide (BiBr₃) | 'B-site' precursor for bismuth-based lead-free PQDs. [80] | ≥98% purity; moisture-sensitive, requires inert atmosphere handling. |
| 1-Octadecene (ODE) | High-boiling non-coordinating solvent for high-temperature synthesis. [82] | 90% purity; often degassed and purified before use. |
| Glutathione (GSH) | Ligand for post-synthetic functionalization; enhances water dispersibility and biocompatibility. [79] | ≥99% purity; used for creating water-dispersible probes. |
| Methyl Acet (MeOAc) | Anti-solvent for purification and ligand exchange processes. [82] | Effectively removes surface ligands without destroying crystal structure. |
Lead-free PQDs synthesized with the OA/OAm ligand pair present a compelling pathway toward clinically viable nanoscale materials. The replacement of lead with "green" metals like bismuth directly addresses the primary toxicity concern, while the versatile OA/OAm system enables the precise synthesis required to achieve acceptable optical performance and stability. However, their clinical translation remains contingent upon overcoming significant challenges. Future research must prioritize rigorous and standardized in-vivo toxicity studies to definitively quantify biosafety profiles. Furthermore, innovative ligand engineering and encapsulation strategies, moving beyond OA/OAm, are essential to enhance the hydrothermal stability and targeting specificity of these materials in complex biological environments. The integration of these advanced lead-free PQDs into multiplexed sensing platforms and their functionalization for targeted therapeutics represent the frontier of research, holding the promise of unlocking their full clinical potential.
Conclusion
The OA/OAm ligand pair is unequivocally a powerful and versatile tool for tailoring the properties of perovskite quantum dots. A meticulously optimized ratio is not merely a synthetic detail but a foundational parameter that directly controls PQD quality, determining their optoelectronic performance, stability, and ultimate suitability for biomedical applications. The convergence of traditional chemical intuition with modern, data-driven optimization strategies provides a robust pathway for synthesizing PQDs with record-high PLQY and enhanced durability. Future progress hinges on developing intelligent, AI-guided synthesis platforms to navigate the complex parameter space more efficiently. For biomedical research, the immediate implications are profound: the ability to produce stable, bright, and biocompatible PQDs opens doors to advanced in vitro diagnostics, highly sensitive biosensors for neurotransmitter detection, and new platforms for bioimaging. The ongoing development of lead-free perovskites synthesized with these optimized ligand strategies will be particularly critical for translating this promising technology into safe and effective clinical tools.
References
- 1. Halide Perovskite Nanostructures: Processing Methods ... [https://www.nature.com/articles/s44310-025-00090-5]
- 2. Recent advances in synthesis and application of perovskite ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC7269082/]
- 3. Enriching conductive capping by alkaline treatment of perovskite quantum dots towards certified 18.3%-efficient solar cells | Nature Communications [https://www.nature.com/articles/s41467-025-63618-5]
- 4. High-quality perovskite quantum dots with excellent ... [https://www.light-am.com/article/doi/10.37188/lam.2025.012]
- 5. / Oleic ligand pair: a versatile combination in the... acid oleylamine [https://pubs.rsc.org/en/content/articlehtml/2022/nh/d2nh00111j]
- 6. Multidentate ligand approach for conjugation of perovskite ... [https://www.sciencedirect.com/science/article/abs/pii/S0021979721009620]
- 7. Control of Emission Color of High Quantum Yield ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC5115379/]
- 8. Thermal tolerance of perovskite quantum dots dependent ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10113222/]
- 9. Fostering the Dense Packing of Halide Perovskite ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10604077/]
- 10. Highly UV Resistant Inch-Scale Hybrid Perovskite Quantum Dot Papers [https://pubmed.ncbi.nlm.nih.gov/32995112/]
- 11. High-performance perovskite quantum dot synthesis ... [https://pubs.rsc.org/en/content/articlelanding/2025/nr/d4nr04942j]
- 12. Review article Perovskite quantum dots: What's next? [https://www.sciencedirect.com/science/article/pii/S2949821X24000577]
- 13. Oleic acid/oleylamine ligand pair: a versatile combination ... [https://pubs.rsc.org/en/content/articlelanding/2022/nh/d2nh00111j]
- 14. Ligand Engineering of Inorganic Lead Halide Perovskite ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11280295/]
- 15. Low-temperature colloidal synthesis of monodisperse ... [https://www.sciencedirect.com/science/article/abs/pii/S0167577X20316402]
- 16. Near unity quantum yield of strongly-confined blue-emitting ... [https://www.sciencedirect.com/science/article/abs/pii/S0925838825032347]
- 17. Effect of PbSO4-Oleate Coverage on Cesium Lead Halide ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC8538890/]
- 18. Facile room-temperature synthesis of highly air‐stable and ... [https://www.sciencedirect.com/science/article/abs/pii/S1369800121004960]
- 19. Pioneering perovskite quantum dot nanosensors for heavy ... [https://pubs.rsc.org/en/content/articlehtml/2025/ra/d5ra06200d?page=search]
- 20. Sequential solid-state multiligand exchange of FAPbI 3 ... [https://pubs.rsc.org/en/content/articlehtml/2025/tc/d5tc00040h]
- 21. Ligand-exchange-assisted printing of colloidal ... [https://www.nature.com/articles/s41467-025-64596-4]
- 22. Toward Green Optoelectronics: Environmental-Friendly ... [https://www.frontiersin.org/journals/energy-research/articles/10.3389/fenrg.2021.666534/full]
- 23. Recent Advances in Ligand Design and Engineering in Lead ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC8224438/]
- 24. Ligand Engineering of Inorganic Lead Halide Perovskite ... [https://www.mdpi.com/2079-4991/14/14/1201]
- 25. Quantifying the Thermodynamics of Ligand Binding to ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC6467082/]
- 26. Growth mechanism of strongly emitting CH 3 NH 3 PbBr ... [https://www.nature.com/articles/s41467-017-00929-2]
- 27. Fluorescence properties and laser-induced enhancement ... [https://www.sciencedirect.com/science/article/abs/pii/S0925346723008261]
- 28. All-Inorganic CsPbBr 3 Perovskite Nanocrystals ... [https://www.mdpi.com/2072-666X/14/7/1332]
- 29. The impact of ligands on the synthesis and application of ... [https://pubs.rsc.org/en/content/articlehtml/2021/ta/d1ta05242j]
- 30. Advancements in Perovskite Nanocrystal Stability Enhancement [https://pmc.ncbi.nlm.nih.gov/articles/PMC10254436/]
- 31. Understanding the Effect of the Synthetic Method and ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10780980/]
- 32. Influence of Binary Ligands in Designing Cesium Lead ... [https://www.mdpi.com/2673-706X/3/4/19]
- 33. Enhancing the quantum yield and stability of CsPbBr3 ... [https://www.sciencedirect.com/science/article/abs/pii/S0022231323007287]
- 34. Partial Ligand Stripping from CsPbBr3 Nanocrystals ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11932522/]
- 35. Enhanced stability and confinement effects of Cs 4 PbBr 6 ... [https://www.sciencedirect.com/science/article/abs/pii/S0925838824004675]
- 36. Flow synthesis and multidimensional parameter screening ... [https://pubs.rsc.org/en/content/articlehtml/2025/na/d4na00839a]
- 37. Compact biocompatible quantum dots via RAFT-mediated ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC2871316/]
- 38. Perovskite Quantum Dots for Emerging Displays [https://pmc.ncbi.nlm.nih.gov/articles/PMC9268187/]
- 39. Flow Synthesis of Metal Halide Perovskite Quantum Dots [https://www.sciencedirect.com/science/article/pii/S2590238520303829]
- 40. Quantum Dot-Enabled Biosensing for Prostate Cancer ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12348777/]
- 41. How to improve the structural stabilities of halide perovskite ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10821855/]
- 42. Exploratory data analysis [https://en.wikipedia.org/wiki/Exploratory_data_analysis]
- 43. Analysis Techniques/Exploratory Data ... [https://rstudio-pubs-static.s3.amazonaws.com/735821_13d7d3a3098b4408a5cb831e10da9511.html]
- 44. Perovskite Quantum Dots Synthesis [https://www.sigmaaldrich.com/US/en/technical-documents/technical-article/materials-science-and-engineering/nanoparticle-and-microparticle-synthesis/synthesis-halide-perovskite-quantum-dots-display-applications?srsltid=AfmBOoqoobNNKpUiNzazKb94GlvSF1MurHKNF3MebUNYuCdsHHnZ8BSO]
- 45. How to improve the structural stabilities of halide perovskite ... [https://nanoconvergencejournal.springeropen.com/articles/10.1186/s40580-024-00412-x]
- 46. Synthetic and Post-Synthetic Strategies to Improve ... [https://www.mdpi.com/2073-4344/11/8/957]
- 47. Homogeneous surface attachment of functional ligands for ... [https://www.sciencedirect.com/science/article/abs/pii/S092583882404578X]
- 48. Machine learning models for accurately predicting ... [https://www.nature.com/articles/s41598-025-08110-2]
- 49. Impact of oleylamine: oleic acid ratio on the morphology ... [https://www.sandia.gov/research/publications/details/impact-of-oleylamine-oleic-acid-ratio-on-the-morphology-of-yttria-nanomater-2017-07-01/]
- 50. DLScanner: A parameter space scanner package assisted ... [https://www.sciencedirect.com/science/article/abs/pii/S0010465525001614]
- 51. Multi-objective artificial-intelligence-based parameter ... [https://www.nature.com/articles/s41598-025-05657-y]
- 52. Oleylamine [https://en.wikipedia.org/wiki/Oleylamine]
- 53. ISOS Protocols for Perovskite Solar Cell Stability Testing [https://www.fluxim.com/research-blogs/isos-protocols-stability-perovskite-solar-cells]
- 54. Dual-function perovskite light-emitting/sensing devices for ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC9684532/]
- 55. Pioneering perovskite quantum dot nanosensors for heavy metal ion... [https://pubs.rsc.org/en/content/articlehtml/2025/ra/d5ra06200d]
- 56. Recent advancements in application of carbohydrate-derived ... [https://bioresourcesbioprocessing.springeropen.com/articles/10.1186/s40643-025-00953-x]
- 57. Biomedical application of carbon quantum dots: A review [https://www.sciencedirect.com/science/article/pii/S2667056924000889]
- 58. CdTe Quantum Dots [https://pqds.alfa-chemistry.com/products/cdte-quantum-dots-4057.html]
- 59. Cost-effective, hydride-free aqueous synthesis of CdTe ... [https://www.sciencedirect.com/science/article/abs/pii/S0022024825001307]
- 60. Current Advances in the Biomedical Applications of ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10454335/]
- 61. Current advances in quantum dots (QDs) and carbon ... [https://www.sciencedirect.com/science/article/pii/S2352492825017167]
- 62. Synthesis of highly fluorescent green carbon quantum dots ... [https://www.nature.com/articles/s41598-025-17535-8]
- 63. Synergetic effect of the surface ligand and SiO2 driven ... [https://www.nature.com/articles/s41598-021-01560-4]
- 64. Ultrasensitive dopamine detection using CsPbBr3-PQD-COF ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12136283/]
- 65. Ultrasensitive dopamine detection with graphene aptasensor ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC9694542/]
- 66. “Two-step” signal amplification for ultrasensitive detection ... [https://www.sciencedirect.com/science/article/abs/pii/S0925400524000376]
- 67. Ultrasensitive detection of dopamine using Au ... [https://pubs.rsc.org/en/content/articlelanding/2024/an/d4an00398e]
- 68. Advances in dopamine electrochemical sensors [https://www.sciencedirect.com/science/article/abs/pii/S0026265X25008896]
- 69. Oleic acid/oleylamine ligand pair: a versatile combination ... [https://pubmed.ncbi.nlm.nih.gov/35770698/]
- 70. Emerging roles of hydrogels, organogels, and their hybrids ... [https://www.nature.com/articles/s44328-025-00055-w]
- 71. Conductive hydrogel-based epidermal electrodes for ... [https://pubs.rsc.org/en/content/articlehtml/2025/tc/d5tc01896j]
- 72. High-performance zinc-ion hydrogel electrolytes based on ... [https://www.sciencedirect.com/science/article/abs/pii/S1005030224006911]
- 73. Highly luminescent CsPbX 3 @MIL-53(Al) ... [https://www.sciencedirect.com/science/article/abs/pii/S0925838824007199]
- 74. Bioprinting of piezoresistive organohydrogel networks for ... [https://www.sciencedirect.com/science/article/pii/S0167779925002124]
- 75. Simple Preparation of Conductive Hydrogels Based on ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12526617/]
- 76. Smart pH-Responsive polymers in biomedical Applications [https://www.sciencedirect.com/science/article/pii/S2468519425005531]
- 77. Based Hydrogel Patch for Long-Term Electrochemical ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12295560/]
- 78. Development of a fluorescence sensor utilising perovskite ... [https://www.sciencedirect.com/science/article/abs/pii/S0272884225046644]
- 79. A lead-free water dispersible GSH-CsCuCl 3 perovskite ... [https://www.sciencedirect.com/science/article/abs/pii/S0167732225019701]
- 80. Eco-friendly synthesis of Cs 3 Bi 2 Br 9 perovskite quantum ... [https://www.sciencedirect.com/science/article/abs/pii/S0013935125002816]
- 81. Lead-Free Cs3Bi2Br9 Perovskite Quantum Dots for Detection of ... [https://www.semanticscholar.org/paper/Lead-Free-Cs3Bi2Br9-Perovskite-Quantum-Dots-for-of-Gao-Chen/87099edcd4d4494b9352bd17f57bce9465625098]
- 82. Ligand exchange engineering of FAPbI3 perovskite quantum ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC9756204/]