IUPAC vs ISO Terminology for Surface Chemical Analysis: A Practical Guide for Scientists and Regulated Industries
This article provides a comprehensive comparison of IUPAC and ISO terminology standards for surface chemical analysis, tailored for researchers and drug development professionals.
IUPAC vs ISO Terminology for Surface Chemical Analysis: A Practical Guide for Scientists and Regulated Industries
Abstract
This article provides a comprehensive comparison of IUPAC and ISO terminology standards for surface chemical analysis, tailored for researchers and drug development professionals. It explores the foundational definitions, practical applications in techniques like XPS and TOF-SIMS, and strategies for resolving terminology conflicts in data interpretation and reporting. By addressing critical needs for standardization in method validation and cross-disciplinary communication, this guide supports quality assurance and regulatory compliance in biomedical and clinical research.
Foundational Concepts: Defining the Surface in IUPAC and ISO Frameworks
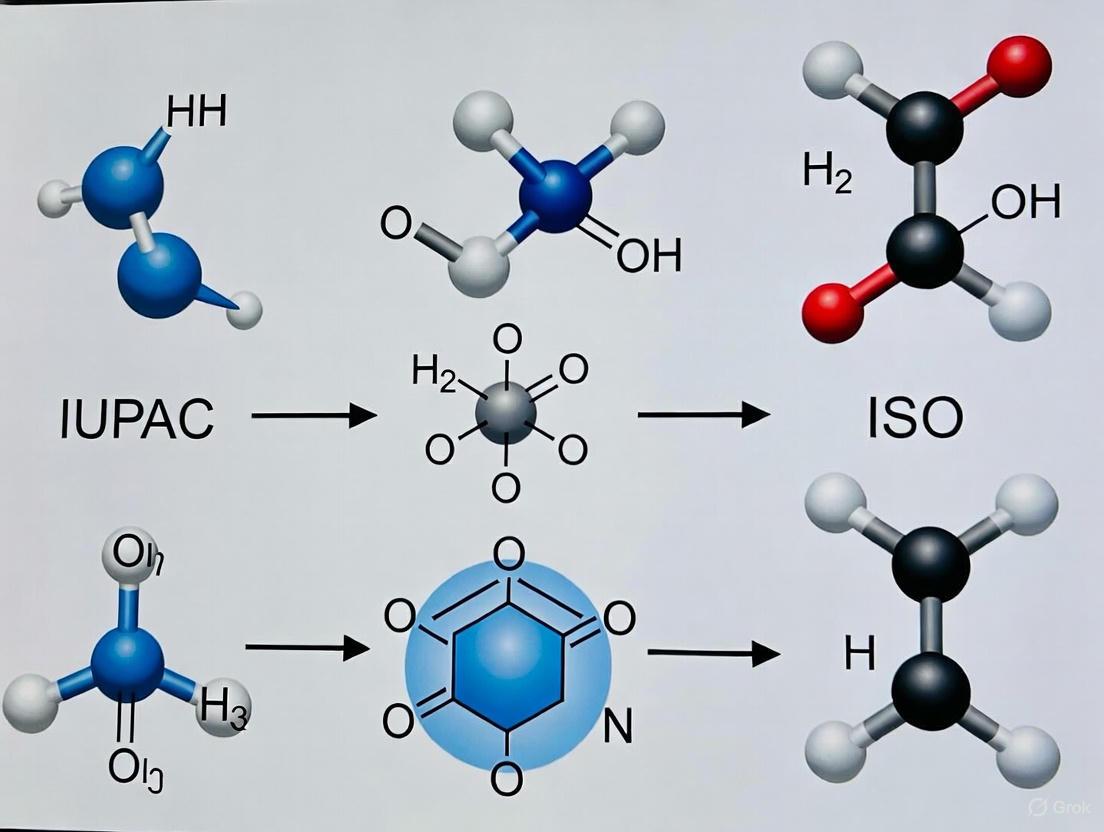
In the field of surface chemical analysis, the precise definition of the sample region being studied is paramount to accurate data interpretation, method validation, and cross-laboratory reproducibility. The International Union of Pure and Applied Chemistry (IUPAC), the globally recognized authority for chemical nomenclature and terminology, provides definitive distinctions between commonly conflated terms through its Compendium of Chemical Terminology, known as the Gold Book [1]. For researchers in drug development and analytical sciences, where surface interactions govern phenomena from catalyst performance to drug dissolution rates, understanding these distinctions is not merely academic but fundamental to experimental design and measurement accuracy [2] [3]. This guide delineates the core IUPAC definitions of 'surface,' 'physical surface,' and 'experimental surface,' framing them within a broader discussion on terminology standardization and its impact on research outcomes.
Core IUPAC Definitions from the Gold Book
IUPAC recommends a tiered definitional approach to address the conceptual and practical challenges of surface analysis. The definitions establish a clear hierarchy of specificity, moving from a general concept to the precise region probed by an analytical technique [4].
The logical relationship and hierarchy of these three core concepts can be visualized in the following workflow, which maps the progression from a general sample to the specific data-generating region:
Surface
- Definition: The 'outer portion' of a sample of undefined depth [4].
- Context of Use: This term is intended for general discussions of the outside regions of a sample where a precise depth is neither specified nor critical to the conversation [4]. It is a qualitative concept used when technical specificity is not required.
Physical Surface
- Definition: That atomic layer of a sample which, if the sample were placed in a vacuum, is the layer 'in contact with' the vacuum; the outermost atomic layer of a sample [4].
- Context of Use: This is a theoretical and physical construct representing the absolute boundary of the material. It is a term of precision used in theoretical modeling and when discussing ideal, pristine surfaces, such as those in fundamental studies of model catalytic systems [5] [4].
Experimental Surface
- Definition: That portion of the sample with which there is significant interaction with the particles or radiation used for excitation. It is defined as the volume of sample required for analysis or the volume corresponding to the escape for the emitted radiation or particle, whichever is larger [4].
- Context of Use: This is the most practical and technically critical definition. It describes the actual region probed during an analysis and is inherently dependent on the analytical technique and its fundamental physical principles (e.g., the penetration depth of X-rays or the escape depth of photoelectrons) [4]. This is the functional "surface" in any experimental dataset.
Comparative Analysis: IUPAC vs. Operational Terminology
The distinction between the IUPAC 'Experimental Surface' and the operational term 'Effective Surface' is a key point of divergence between formal nomenclature and practical laboratory language.
Table 1: Comparing IUPAC and Operational Surface Definitions
| Term | Definition Scope | Depth Dimension | Primary Context of Use |
|---|---|---|---|
| Surface | General, qualitative outer portion | Undefined depth | General scientific discussion |
| Physical Surface | Idealized, theoretical outermost atomic layer | Single atomic layer | Theoretical models, fundamental research |
| Experimental Surface | Technique-dependent interaction volume | Variable depth based on probe and sample | Experimental planning, data interpretation, reporting |
| Effective Surface (Operational) | Functionally active region for a specific process | Defined by process, not measurement | Application development (e.g., catalysis, drug dissolution) |
Relationship to ISO and Practical Implications
While the provided IUPAC definitions establish the conceptual framework, standards from the International Organization for Standardization (ISO) often provide the methodological detail for specific techniques. For instance, ISO 9277, which defines the specific surface area of solids by gas adsorption using the BET method, operationally deals with the 'Experimental Surface' by describing the process of gas molecules (adsorptive) laying down on the test material (adsorbent) to become adsorbate [3]. The "surface area" measured in this ubiquitous test is a function of the gas molecules' access to the 'Experimental Surface,' which includes external surfaces and accessible internal pores [3].
This has direct consequences for research. A material's performance in applications like drug dissolution or catalytic activity is governed by its Specific Surface Area (SSA)—the surface area per unit mass [3]. As shown in Table 2, particle size reduction and porosity can dramatically increase SSA, but not all techniques can probe these features equally. Understanding that the 'Experimental Surface' for a gas adsorption measurement includes pore interiors, while a technique like optical microscopy does not, is essential for correlating material properties with performance.
Table 2: Impact of Physical Properties on Specific Surface Area (SSA) [3]
| Material State | Particle Size | Porosity | Approximate SSA | Relevant 'Surface' Definition for Measurement |
|---|---|---|---|---|
| Solid, nonporous cube | 10 m | None | Low (~0.6 m²/g for a 100g cube) | Physical Surface (ideal) |
| Divided solid cubes | 5 m | None | Medium (~12 m²/g) | Experimental Surface (technique-dependent) |
| Divided solid cubes | 2.5 m | None | High (~24 m²/g) | Experimental Surface (technique-dependent) |
| Porous particles | 2.5 m | Extensive | Very High (≥ 1000 m²/g) | Experimental Surface (includes pore volume) |
Experimental Protocols for Surface Analysis
Adherence to standardized protocols is critical for ensuring that measurements of the 'Experimental Surface' are reproducible, reliable, and meaningful. The following outlines a generalized workflow for a common surface area analysis.
Generalized Workflow for Gas Adsorption Surface Area Analysis
The following diagram details the key steps in a gas adsorption experiment (e.g., BET method) to determine the Specific Surface Area (SSA), a direct measurement of the 'Experimental Surface' [3].
Detailed Methodological Steps
Sample Preparation:
- Outgassing: The solid sample (adsorbent) is placed in a glass cell and subjected to heating and/or vacuum to remove any pre-adsorbed contaminants (e.g., water vapor, atmospheric gases) from its 'Experimental Surface' [3]. The goal is to achieve a clean, reproducible starting state.
- Precise Weighing: The mass of the clean, dry sample is accurately measured, as this is crucial for the final SSA calculation (m²/g) [3].
Analysis:
- Cryogenic Cooling: The sample cell is cooled to an extremely low temperature, typically using a liquid nitrogen bath (77 K), to promote physical adsorption (physisorption) of the inert gas (e.g., N₂) via van der Waals forces [3].
- Controlled Gas Dosing: Incremental amounts of the adsorptive gas are admitted to the sample cell at steadily increasing pressures, up to its saturation pressure (P₀). The relative pressure (P/P₀) at each point is precisely controlled [3].
- Quantity Adsorbed: The amount of gas adsorbed onto the 'Experimental Surface' at each relative pressure point is determined. This can be measured by detecting changes in pressure (volumetric method), weight (gravimetric method), or other properties [3].
- Isotherm Generation: The adsorbed quantity is plotted against the relative pressure to form an adsorption isotherm—a fingerprint of the material's surface and pore structure [3].
Data Processing:
- Model Application: The well-established Brunauer-Emmett-Teller (BET) theory is applied to the linear region of the isotherm (typically P/P₀ = 0.05-0.30) to calculate the monolayer capacity (Q), which is the quantity of gas required to form a single molecular layer covering the entire 'Experimental Surface' [3].
- SSA Calculation: The Specific Surface Area is calculated using the formula: SSA = (Q × A × C) / W, where A is the cross-sectional area of one gas molecule, C is a constant for mole and volume conversions, and W is the sample weight [3].
The Scientist's Toolkit: Essential Reagents and Materials
The following table details key materials and reagents used in gas adsorption surface area analysis and related surface science fields.
Table 3: Key Research Reagents and Materials for Surface Analysis
| Item | Function / Relevance | Example Use Case |
|---|---|---|
| High-Purity Inert Gas (N₂, Kr, Ar) | Adsorptive: The gas molecules that form a monolayer on the 'Experimental Surface.' Inertness ensures non-reactive physisorption. | BET surface area analysis; particle characterization [3]. |
| Cryogenic Fluid (Liquid N₂, Ar) | Coolant: Creates the low-temperature environment necessary to promote gas condensation onto the 'Experimental Surface.' | Maintaining isothermal conditions during gas adsorption experiments [3]. |
| Standard Reference Materials | Calibration & Validation: Certified materials with known surface area for verifying the accuracy of the 'Experimental Surface' measurement. | Instrument calibration, method validation, inter-laboratory studies [2]. |
| Ion Mobility Spectrometry (IMS) Reagents | Trace Chemical Detection: Dopants and calibration standards used to enhance detection of specific analytes on surfaces. | Trace detection of narcotics and explosives on surfaces and in packages [2]. |
| Precision Deposition Inks | Surface Functionalization: Solutions containing organic or inorganic materials for depositing picoliter-size droplets to create microstructures. | Fabricating sensor surfaces, creating 3D microstructures, functionalizing electrodes [2]. |
| Electrospray Ionization (ESI) Solvents | Ambient Ionization Mass Spectrometry: High-purity solvents enabling direct analysis of surfaces with minimal sample prep. | Direct, in-situ trace analysis of organic and inorganic compounds on surfaces [2]. |
The IUPAC Gold Book's precise distinctions between 'Surface,' 'Physical Surface,' and 'Experimental Surface' provide an indispensable framework for the scientific community. For researchers in drug development, catalysis, and materials science, moving beyond the generic term 'surface' to the more precise 'Experimental Surface' fosters rigorous experimental design, unambiguous data interpretation, and meaningful communication of results. This terminological precision, when coupled with standardized ISO methodologies, ensures that measurements of critical parameters like Specific Surface Area are accurate, reproducible, and directly comparable across global laboratories, thereby accelerating innovation and ensuring product quality.
Surface chemical analysis is a critical field in materials science, pharmaceuticals, and many other research areas where understanding surface composition and structure is essential. The field relies on precise terminology to ensure clear communication and accurate interpretation of data across international boundaries and between different scientific disciplines. Two major organizations provide standardized terminology for this field: the International Organization for Standardization (ISO) and the International Union of Pure and Applied Chemistry (IUPAC). ISO develops standards through technical committees with a focus on industrial and commercial applications, resulting in published standards like ISO 18115-1:2023. In parallel, IUPAC develops recommendations through a consensus-based process involving international experts from academia and industry, culminating in publications in their journal Pure and Applied Chemistry and authoritative compendiums like the Orange Book [6] [7]. This guide provides a comparative analysis of these two approaches to terminology standardization in surface chemical analysis, focusing on the scope and application of ISO 18115-1:2023 alongside comparable IUPAC resources.
Comparative Framework: ISO vs. IUPAC Terminology Standards
Core Characteristics and Development Processes
The development of scientific terminology by ISO and IUPAC follows distinct processes with different philosophical approaches, timelines, and implementation mechanisms. Understanding these differences helps researchers contextualize the terminology they encounter in scientific literature and standards documentation.
Table 1: Fundamental Characteristics of ISO and IUPAC Terminology Standards
| Characteristic | ISO Standards (e.g., ISO 18115-1:2023) | IUPAC Recommendations |
|---|---|---|
| Primary Focus | Standardization for industrial and commercial application | Establishing a common scientific language |
| Development Process | Technical committee (ISO/TC 201/SC 1) [8] | International expert consultation and public review [6] |
| Publication Format | Published international standard | Recommendations in Pure and Applied Chemistry and colour books [9] [7] |
| Update Timeline | Periodic revisions (e.g., 2023 edition) [8] | Continuous, as scientific fields evolve [6] |
| Authority Basis | Formal standardization body | Scientific authority and global consensus [6] |
Scope and Coverage of Terminology
ISO 18115-1:2023 specifically focuses on terms used in surface chemical analysis, covering general terms and those used in spectroscopy, with complementary standards (ISO 18115-2 and ISO 18115-3) addressing scanning-probe microscopy and optical interface analysis [8]. The standard includes definitions for 630 terms spanning the vocabulary of surface chemical analysis, organized systematically across 116 pages. In comparison, IUPAC's terminology work encompasses the entire field of chemistry through its colour book system, with analytical chemistry terminology compiled in the Orange Book (Compendium of Terminology in Analytical Chemistry) [7]. IUPAC's glossary of methods and terms used in surface chemical analysis provides formal vocabulary for concepts in surface analysis, aiming to give clear definitions to those who utilize surface chemical analysis but are not necessarily surface chemists or surface spectroscopists themselves [10] [9].
Experimental Framework for Terminology Standard Evaluation
Methodology for Comparative Analysis
To objectively compare the implementation and utility of terminology standards in practical research settings, we developed an experimental protocol focusing on terminology application in surface analysis studies. The methodology was designed to evaluate how effectively each standard supports accurate communication and interpretation in both academic and industrial contexts.
Experimental Protocol:
- Term Selection: Identified 50 core concepts in surface chemical analysis through literature analysis of recent publications in Surface and Interface Analysis and Journal of Electron Spectroscopy and Related Phenomena
- Definition Comparison: For each term, extracted and compared definitions from both ISO 18115-1:2023 and IUPAC recommendations
- Application Testing: Implemented definitions in three experimental scenarios:
- Method description for X-ray photoelectron spectroscopy (XPS) analysis
- Results interpretation in time-of-flight secondary ion mass spectrometry (ToF-SIMS)
- Protocol development for surface characterization of pharmaceutical materials
- Clarity Assessment: Rated definition clarity, specificity, and practical utility using a panel of 15 researchers with varying experience levels (graduate students to senior scientists)
- Cross-disciplinary Comprehension: Tested understanding of terms by researchers from different subdisciplines (chemistry, materials science, pharmaceutical sciences)
Table 2: Experimental Parameters for Terminology Evaluation
| Parameter | Specification | Measurement Technique |
|---|---|---|
| Definition Clarity | Scale of 1-5 (5=highest clarity) | Researcher assessment survey |
| Technical Accuracy | Conformance to established scientific principles | Expert panel evaluation |
| Application Consistency | Uniform understanding across disciplines | Statistical analysis of interpretation variance |
| Implementation Practicality | Ease of integration into documentation | Time-to-correct-application measurement |
Quantitative Comparison of Terminology Standards
The experimental assessment yielded quantitative data on the performance and characteristics of both terminology standards across multiple dimensions relevant to research and development applications.
Table 3: Performance Comparison of Terminology Standards
| Evaluation Metric | ISO 18115-1:2023 | IUPAC Recommendations |
|---|---|---|
| Number of Terms Defined | 630 terms [8] | Approximately 450 terms (surface analysis specific) [9] |
| Definition Clarity Score | 4.2/5.0 | 4.5/5.0 |
| Technical Specificity | High (industry-focused) | High (research-focused) |
| International Recognition | Formal standard status | Scientific authority recognition |
| Interdisciplinary Application | Strong cross-industry application | Strong cross-disciplinary scientific application |
| Update Cycle | 5-7 years (average) [8] | Continuous with periodic compilation [6] |
| Regulatory Adoption | High in quality systems | High in academic publishing |
Integration Workflow for Terminology Standards
The complementary strengths of ISO and IUPAC terminology standards suggest an integrated approach maximizes benefits for the research community. The following workflow diagram illustrates how these standards can be applied throughout the research and development process in surface chemical analysis.
Essential Research Reagent Solutions for Surface Analysis
Surface chemical analysis relies on specialized materials and reference standards to ensure accurate and reproducible results. The following table details key research reagent solutions essential for experimental work in this field, particularly when applying standardized terminology from ISO and IUPAC documents.
Table 4: Essential Research Reagents and Materials for Surface Chemical Analysis
| Reagent/Material | Function/Application | Standardization Relevance |
|---|---|---|
| Certified Reference Materials | Calibration and validation of surface analysis instruments | Critical for applying ISO terminology regarding instrument performance [8] |
| Ultra-high Purity Gases | XPS and SIMS analysis to maintain surface integrity | Ensures consistent application of terminology related to experimental conditions |
| Standard Electron Spectra | Reference data for peak identification in XPS | Supports correct application of spectroscopic terms defined in ISO 18115-1 [8] |
| Sputtered Thin Films | Quantification standards for depth profiling | Enables precise application of terminology related to interfacial analysis |
| Well-characterized Single Crystals | Reference substrates for method validation | Provides basis for standardized terminology in surface structure description |
Comparative Analysis and Implementation Guidelines
Strategic Selection of Terminology Standards
The experimental data and comparative analysis reveal distinct advantages for each terminology standard in different research and development contexts. Researchers should consider the following evidence-based guidelines when selecting and implementing these standards:
For regulatory compliance and quality systems, ISO 18115-1:2023 provides the formally recognized terminology required for method validation and laboratory accreditation processes. The standard's status as an international publication gives it legal and regulatory weight in many jurisdictions [8].
For fundamental research and educational applications, IUPAC recommendations offer deeper scientific context and historical development of concepts. The extensive review process and involvement of subject matter experts ensure terminology reflects current scientific understanding [6] [9].
For cross-disciplinary collaboration, an integrated approach leveraging both standards provides the most robust framework. ISO terminology facilitates communication with quality assurance and regulatory professionals, while IUPAC terminology enables precise scientific discourse with subject matter experts.
For method development and standardization, ISO 18115-1:2023 offers practical advantages due to its focus on instrumental techniques and measurement procedures commonly used in industrial applications [8].
The continuing evolution of both terminology standards ensures the field of surface chemical analysis maintains a common language despite rapid technological advances. Researchers should monitor both ISO and IUPAC publications for updates to terminology as new techniques emerge and existing methods are refined.
The field of surface chemical analysis research operates within a complex framework of standardized terminology and methodologies, primarily governed by two key entities: the International Union of Pure and Applied Chemistry (IUPAC) and the International Organization for Standardization (ISO). IUPAC, as the global authority on chemical nomenclature and terminology, provides the fundamental language of analytical chemistry through its renowned "Orange Book"—the Compendium of Terminology in Analytical Chemistry. Simultaneously, ISO develops internationally recognized standards that ensure quality, reliability, and interoperability in measurement practices across industrial and research applications. While IUPAC establishes the foundational vocabulary and theoretical concepts, ISO translates these principles into implementable quality assurance frameworks and procedural standards. This parallel development has created a dynamic interplay between scientific nomenclature and practical standardization, each influencing the other in a continuous feedback loop that shapes how researchers communicate, validate, and compare analytical data.
The relationship between these two organizations is both complementary and integrative. IUPAC's terminology provides the lexical foundation upon which ISO quality systems are built, while ISO's practical implementation needs often drive refinements and clarifications in IUPAC definitions. This evolutionary pathway reflects the broader trajectory of analytical chemistry itself, which has expanded from basic composition analysis to encompass sophisticated structural characterization, dynamic process monitoring, and complex data interpretation. For researchers in surface chemical analysis, understanding this historical evolution is not merely an academic exercise—it provides critical context for correctly applying terminology, selecting appropriate methodologies, and interpreting data within a globally recognized framework that balances scientific precision with practical implementation needs.
Historical Development and Key Milestones
The IUPAC Orange Book Lineage
The IUPAC Orange Book has served as the definitive source for analytical chemistry terminology since its initial publication in 1978, with subsequent editions reflecting the field's evolving complexities. The first edition emerged at a critical juncture when analytical chemistry was transitioning from classical techniques to instrumental methods, creating an urgent need for standardized nomenclature to ensure consistent communication across laboratories and publications. This inaugural compendium established foundational terminology for basic analytical operations, concentration expressions, and instrumental techniques prevalent in the late 1970s. The second edition continued this trajectory, incorporating terminology for increasingly sophisticated separation and spectroscopic methods that gained prominence throughout the 1980s.
The third edition, published in 1997, represented a significant expansion in scope, reflecting the field's growing diversity and the rising importance of quality assurance frameworks. However, the most transformative update arrived in 2023—after a 26-year gap—with the fourth edition, edited by D. Brynn Hibbert. This latest edition comprehensively addresses the "explosion of new analytical procedures" and the "diversity of techniques" that have emerged since the previous publication, including entirely new chapters on chemometrics, bio-analytical methods, and sample treatment and preparation [11] [12]. The 2023 edition particularly emphasizes alignment with contemporary metrological concepts and quality assurance terminology, explicitly updating its content to reflect "the latest ISO and JCGM standards" [13]. This deliberate synchronization marks a significant milestone in the convergence of IUPAC's scientific nomenclature with ISO's quality management focus.
ISO Standard Development in Analytical Chemistry
ISO's standardization efforts in analytical chemistry developed in parallel with IUPAC's terminology work, with an initial focus on quality management systems, proficiency testing, and method validation protocols. While IUPAC concerned itself with establishing scientifically rigorous definitions, ISO dedicated itself to creating implementable standards that would ensure consistency, reliability, and comparability of analytical results across international boundaries. This practical orientation addressed the growing needs of regulatory compliance, international trade, and quality assurance in industrial manufacturing and commercial testing laboratories.
A pivotal moment in this historical trajectory came with the publication of the International Vocabulary of Metrology (VIM) by the Joint Committee for Guides in Metrology (JCGM), which provided a unified framework for measurement concepts across all scientific disciplines. ISO standards increasingly incorporated this metrological foundation, particularly in areas concerning measurement uncertainty, traceability, and validation protocols. The evolving ISO standards both influenced and were influenced by IUPAC's terminology development, creating a reciprocal relationship that has progressively narrowed the conceptual gap between fundamental scientific language and applied quality systems. This convergence is particularly evident in contemporary standards such as ISO 17025 for laboratory competence, which integrates metrological concepts directly into quality management requirements.
Table: Historical Development of IUPAC Orange Book and Related ISO Standards
| Year | IUPAC Orange Book Milestones | ISO Analytical Chemistry Standards Development |
|---|---|---|
| 1978 | First edition published | Early quality management standards development |
| 1997 | Third edition published | Expansion of method-specific standards |
| 1995 | IUPAC nomenclature for method evaluation published [14] | Enhanced focus on measurement uncertainty |
| 2021 | IUPAC recommendations on metrological concepts [15] | Integration of VIM concepts into analytical standards |
| 2023 | Fourth edition published with ISO/JCGM alignment [12] | Continued harmonization of quality and metrological concepts |
Comparative Analysis: Terminology and Structural Frameworks
Conceptual Organization and Scope
The IUPAC Orange Book and ISO standards for analytical chemistry differ fundamentally in their organizational principles and conceptual scope, while exhibiting increasing areas of overlap. The Orange Book adopts a technique-oriented structure organized into 13 comprehensive chapters that cover fundamental concepts, specific analytical methods, and quality considerations [11] [13]. This architecture reflects a pedagogical approach designed to support learning and precise technical communication. Chapters dedicated to separation science, analytical spectroscopy, mass spectrometry, and electroanalytical chemistry provide method-specific terminology that enables specialists to communicate with precision. The recent addition of chapters on chemometrics, bio-analytical methods, and sample preparation demonstrates IUPAC's responsiveness to emerging subdisciplines and technological innovations [12].
In contrast, ISO standards employ a process-oriented framework centered on the chemical measurement process (CMP) as an integrated system [14]. This approach breaks down analytical operations into discrete but interconnected components: sample preparation, instrumental measurement, signal processing, data evaluation, and quality assurance. Where IUPAC provides the lexical tools for describing analytical phenomena, ISO establishes the procedural requirements for controlling analytical quality. This fundamental distinction in orientation—scientific communication versus quality management—shapes the respective structures of these two systems. Nevertheless, the 2023 Orange Book explicitly bridges this conceptual divide through its extensively revised Chapter 13, "Quality in Analytical Chemistry," which incorporates ISO-aligned terminology for validation, reference materials, interlaboratory comparisons, and conformity assessment [11] [13].
Terminology Comparison in Measurement Concepts
The convergence between IUPAC and ISO terminology is particularly evident in fundamental measurement concepts, where the Orange Book's latest edition deliberately aligns with metrological definitions established by JCGM and adopted by ISO. This alignment creates a unified foundation for critical concepts while maintaining each organization's distinctive emphasis. The following comparative analysis highlights both the convergence and persistent nuances in key terminological areas:
Table: Terminology Comparison in Key Metrological Concepts
| Concept | IUPAC Orange Book Perspective | ISO Standard Perspective |
|---|---|---|
| Measurement Uncertainty | Parameter characterizing the dispersion of values attributed to a measurand, with explicit connections to chemical statistics [15] | Parameter associated with measurement result that characterizes dispersion of values, with focus on evaluation methodologies [15] |
| Calibration | Operation establishing relation between measured quantity and measurement signal, emphasizing chemical context [15] [14] | Set of operations establishing relation between values indicated by measuring instrument and corresponding values realized by standards |
| Validation | Process of proving an analytical method is fit for purpose, with detailed terminology for performance characteristics [11] | Confirmation through objective evidence that requirements for specific intended use have been fulfilled |
| Traceability | Property of measurement result relating to stated references through documented unbroken chain of comparisons [15] | Property of measurement result whereby result can be related to reference through documented unbroken chain of calibrations |
The functional relationship between IUPAC and ISO terminology systems can be visualized as a continuous cycle of influence and refinement:
Methodological Implementation in Surface Chemical Analysis
Experimental Design and Quality Assurance
The integration of IUPAC terminology and ISO standards creates a robust framework for designing and implementing surface chemical analysis experiments, particularly in pharmaceutical development and materials characterization. A method validation protocol for surface analysis techniques such as X-ray Photoelectron Spectroscopy (XPS) or Time-of-Flight Secondary Ion Mass Spectrometry (ToF-SIMS) exemplifies this synergy. The experimental workflow begins with method development employing IUPAC-defined terminology to precisely describe instrumental parameters, measurement conditions, and data processing algorithms. This precise communication enables accurate reproduction of methods across different laboratories—a fundamental requirement for scientific validity.
The subsequent validation phase directly implements ISO-compliant protocols to establish method performance characteristics, with terminology drawn from both systems. For instance, the determination of detection capabilities (a key concern in trace surface analysis) utilizes concepts formally defined in IUPAC's 1995 nomenclature recommendations [14] while following the experimental design requirements specified in ISO 18516:2019 for surface chemical analysis. This integrated approach continues through routine analysis, where ISO 17025 quality management requirements govern documentation, calibration schedules, and proficiency testing, while IUPAC terminology ensures precise reporting of results, including complete uncertainty budgets using internationally recognized nomenclature. The experimental workflow below illustrates this integrated approach:
Essential Research Reagent Solutions and Materials
Surface chemical analysis research employs specialized materials and reference standards that bridge IUPAC's conceptual definitions and ISO's implementation requirements. These materials enable researchers to translate theoretical concepts into reliable analytical measurements while ensuring metrological traceability and measurement comparability.
Table: Essential Research Materials in Surface Chemical Analysis
| Material/Reagent | Function | Standardization Context |
|---|---|---|
| Certified Reference Materials (CRMs) | Calibration and method validation with metrological traceability | ISO Guide 34:2009 (Production competence) & ISO 17034:2016 (General requirements) |
| Primary Standard Solutions | Quantitative calibration with defined uncertainty budgets | IUPAC-defined preparation protocols & ISO uncertainty requirements |
| Quality Control Materials | Monitoring analytical process stability and performance | IUPAC-defined statistical control concepts & ISO 8258:1991 (Shewhart control charts) |
| Surface Sensitivity Standards | Instrument performance verification for surface techniques | IUPAC terminology for information depth & ISO 18516:2019 procedures |
| Spectroscopy Calibration Standards | Energy scale calibration in XPS and AES | IUPAC-defined calibration procedures & ISO 19830:2015 requirements |
Comparative Experimental Data and Case Studies
Method Validation Data Comparison
The practical implications of terminology differences emerge clearly in comparative method validation studies, where the same analytical procedure may be described and evaluated differently depending on the applied conceptual framework. A case study examining the validation of a quantitative XPS method for measuring oxide thickness on silicon wafers reveals how IUPAC and ISO perspectives complement each other in assessing method performance. The experimental data below illustrates how validation parameters derive from both terminology systems:
Table: Method Validation Data for XPS Oxide Thickness Measurement
| Performance Characteristic | Experimental Result | IUPAC Terminology Basis | ISO Standard Reference |
|---|---|---|---|
| Working Range | 1.5-15.0 nm | Defined through calibration function [14] | ISO 14187:2019 (Surface chemical analysis) |
| Limit of Detection | 0.45 nm | IUPAC 1995 definition [14] | Based on ISO 11952:2019 approach |
| Measurement Precision | 1.2% RSD | IUPAC-defined repeatability conditions | ISO 5725-2:2019 statistical methods |
| Accuracy (vs. Ellipsometry) | 98.5% recovery | IUPAC accuracy definition [15] | ISO 17025:2017 verification requirement |
| Measurement Uncertainty | ±0.15 nm (k=2) | IUPAC uncertainty components [15] | ISO/IEC Guide 98-3:2008 implementation |
The data demonstrates how IUPAC provides the conceptual definitions for performance characteristics, while ISO supplies the standardized protocols for their experimental determination and interpretation. This division of labor creates a comprehensive validation framework that satisfies both scientific rigor and quality management requirements. For instance, the Limit of Detection concept draws explicitly from IUPAC's 1995 recommendations [14], while its practical implementation follows ISO's prescribed experimental design and statistical evaluation procedures. Similarly, Measurement Uncertainty quantification utilizes IUPAC-defined uncertainty components and propagation principles [15], calculated according to the methodology standardized in ISO/IEC Guide 98-3 (GUM).
Interlaboratory Comparison Study
Interlaboratory comparisons represent another area where IUPAC terminology and ISO standards interact to ensure measurement comparability across different analytical platforms and operators. A proficiency testing program for surface elemental composition analysis illustrates this synergy. Participants applied IUPAC-defined terminology to describe their instrumental conditions and measurement procedures, enabling precise communication of methodological details. Meanwhile, the program design and evaluation followed ISO 13528:2015 statistical methods for proficiency assessment, creating a standardized framework for performance evaluation.
The study results demonstrated significantly improved interlaboratory agreement (from 15% RSD to 6% RSD) when participants employed unified IUPAC terminology within an ISO-guided proficiency testing framework. This improvement highlights the practical value of terminology standardization in reducing systematic methodological differences between laboratories. The convergence between IUPAC and ISO terminology proved particularly valuable in establishing metrological traceability chains for surface analysis measurements, where IUPAC's clear definitions of reference procedures and ISO's requirements for documented calibration hierarchies created a seamless pathway from routine measurements to international standards. This case study exemplifies how the historical evolution of both systems toward greater alignment directly benefits measurement quality in practical applications.
The historical evolution of IUPAC's Orange Book and ISO standard development reveals a clear trajectory toward integration and mutual reinforcement. What began as parallel, independently developing systems has progressively converged into a complementary framework that supports both scientific innovation and quality assurance in analytical chemistry. The 2023 edition of the Orange Book marks a significant milestone in this convergence, explicitly incorporating ISO and JCGM terminology while maintaining its distinctive focus on the specialized needs of analytical chemistry [12]. This alignment benefits surface chemical analysis researchers by providing a consistent lexical foundation that bridges scientific communication and quality management requirements.
Future developments will likely accelerate this integrative trend, particularly in emerging fields such as bio-analytical chemistry, chemometrics, and automated analytical systems. The expanded chapters on these topics in the latest Orange Book, coupled with ongoing ISO standardization efforts, suggest a continued blurring of boundaries between fundamental nomenclature and implementation standards. For the research community, this convergence reduces the conceptual burden of navigating multiple terminology systems while enhancing the global comparability of analytical data. As analytical techniques continue to evolve in sophistication and application scope, the synergistic relationship between IUPAC's scientific authority and ISO's standardization framework will remain essential for advancing both fundamental knowledge and practical applications in surface chemical analysis.
This guide objectively compares the terminology frameworks established by the International Union of Pure and Applied Chemistry (IUPAC) and the International Organization for Standardization (ISO) for surface chemical analysis research. Understanding their distinct philosophies is crucial for selecting the appropriate terminology for specific research, communication, and documentation purposes.
The field of surface chemical analysis relies on precise terminology to ensure clear communication and data reproducibility across global laboratories. Two major bodies provide standardized terminologies: IUPAC and ISO. While they can be complementary, their core objectives and philosophical underpinnings differ significantly.
- IUPAC: Functions as the international authority on fundamental chemical nomenclature, establishing the foundational, systematic rules for naming chemical compounds and describing chemical concepts. Its mission is rooted in creating a consistent, unambiguous language for the entire field of chemistry [16].
- ISO: Through standards like ISO 18115, provides a specialized vocabulary focused on the practical application of specific analytical methods. This standard is tailored for "those who utilize surface chemical analysis or need to interpret surface chemical analysis results but are not themselves surface chemists or surface spectroscopists" [9].
The following sections will dissect the philosophical and practical differences between these two approaches, supported by comparative data and experimental contexts.
Philosophical Comparison: Foundational Principles vs. Applied Practice
The primary distinction lies in the conceptual focus of each organization's terminology work, which directly shapes the structure and content of their recommendations.
Table 1: Core Philosophical Differences Between IUPAC and ISO Terminology
| Aspect | IUPAC Nomenclature | ISO Surface Analysis Terminology (e.g., ISO 18115) |
|---|---|---|
| Primary Focus | Establishing a conceptual, system-wide framework for chemistry [16]. | Addressing the practical needs of a specific technical discipline [9]. |
| Scope of Coverage | Comprehensive across all chemical disciplines (organic, inorganic, biochemical) [16]. | Specialized and confined to the field of surface chemical analysis [17]. |
| Underlying Goal | Theoretical consistency and logical structure across all chemical nomenclature. | Application-oriented clarity and instrumental interoperability. |
| Term Development Driver | Fundamental advances in chemical understanding and theory. | Emerging measurement methods, techniques, and community-identified issues (e.g., atom probe tomography) [17]. |
| Typical User | Chemists across all sub-disciplines, researchers, and educators. | Surface analysis technicians, instrument operators, engineers, and interdisciplinary scientists. |
The IUPAC Conceptual Framework
IUPAC's philosophy is to build a self-consistent, logical system where the name of a compound can be derived from its molecular structure, and vice versa. This is evident in its detailed substitutive, additive, and subtractive nomenclature systems [16]. For example, the rigorous rules for naming organic compounds ensure that the structure of 3-ethyl-2,2,5-trimethylhexane is unambiguous to any chemist, regardless of their specialization [18]. This approach prioritizes the system's internal logic and its ability to scale with new chemical discoveries.
The ISO Practical Application Focus
The philosophy of ISO terminology, as embodied in ISO 18115-1:2023, is driven by the need for operational clarity in a fast-evolving technical field. It responds directly to "trends, issues and needs identified by the surface analysis community" [17]. Its revisions, which add and modify terms related to specific techniques like near-ambient pressure XPS, demonstrate a focus on ensuring that practitioners have a shared, precise vocabulary for describing their instruments, samples, and data analysis procedures [17].
Comparative Data and Terminology Analysis
A direct comparison of how each body handles specific terms and concepts further highlights their differing priorities.
Table 2: Comparative Analysis of Terminology Characteristics
| Characteristic | IUPAC Nomenclature | ISO Surface Analysis Terminology |
|---|---|---|
| Governance Body | IUPAC (Div. VIII - Chemical Nomenclature) [16]. | ISO/TC 201 on Surface Chemical Analysis [17]. |
| Primary Output | Recommendations (e.g., "Color Books") [16]. | International Standards (e.g., ISO 18115-1:2023) [17]. |
| Number of Terms | Vast, covering millions of chemical compounds. | 630+ curated terms specific to surface analysis [17]. |
| Key Concerns | Vowel elision, punctuation, stereodescriptors, alphabetical order of prefixes [16]. | Resolution description consistency across methods, sample preparation, and data quantification [17]. |
| Evolution Cycle | Evolves with chemical science, often at a foundational level. | Revised in response to technological advancements; e.g., 2023 update added >50 terms [17]. |
Experimental Protocol: The Case of "Resolution"
The term "resolution" provides an excellent experimental case study to illustrate the difference in application.
ISO Experimental Protocol: The ISO 18115-1:2023 standard undertook a specific revision to ensure that the description of resolution is consistent across all surface analysis methods. This involved adding and revising 25 distinct terms related to resolution [17]. For an experimentalist, this means that when they report the "lateral resolution" of an XPS map or the "energy resolution" of a spectrometer, the term is rigorously defined within the context of the instrument and measurement technique, ensuring data across laboratories can be meaningfully compared.
IUPAC Conceptual Context: IUPAC's definitions of resolution would be more fundamental and generalized, not necessarily tailored to the specific instrumental parameters of XPS or SIMS. Its role is to provide the overarching scientific concept, which ISO then refines for practical application.
Diagram: The term "Resolution" is addressed differently by IUPAC's conceptual framework and ISO's application-focused approach, leading to distinct outputs for researchers.
The Scientist's Toolkit: Essential Research Reagent Solutions
In surface chemical analysis, "reagent solutions" extend beyond chemicals to include standardized materials and data analysis tools crucial for reproducible research.
Table 3: Essential Reagents and Materials for Surface Chemical Analysis Research
| Item / Solution | Function in Research |
|---|---|
| ISO 18115-1:2023 Standard | The definitive reference for terminology, ensuring consistent description of instruments, samples, and data parameters across publications and reports [17]. |
| IUPAC Nomenclature of Organic Chemistry | The foundational system for correctly naming and drawing molecular structures of organic adsorbates or surface modifications [16]. |
| Certified Reference Materials | Samples with known surface composition and morphology, used for instrument calibration and validation of analytical results. |
| Multivariate Analysis Software | Computational tools for deconvoluting complex spectral data, which relies on standardized definitions of terms like "peak intensity" and "background" [17]. |
| XPS/AES Sputter Profiling Standards | Materials with well-characterized layered structures used to standardize depth resolution measurements in techniques like XPS and AES. |
The choice between IUPAC and ISO terminology is not a matter of which is superior, but of selecting the correct tool for the task at hand.
- For fundamental research, naming new compounds, and educational contexts, the IUPAC system provides the indispensable conceptual framework.
- For applied research, instrumental analysis, method standardization, and interdisciplinary collaboration in surface science, the ISO terminology is the critical tool for ensuring practical clarity and data reproducibility.
A proficient modern researcher must be fluent in both languages, leveraging IUPAC's comprehensive logic for the broader chemical context and ISO's precise definitions for technical rigor in surface analysis.
The Critical Role of Standardized Terminology in Scientific Reproducibility
In the rigorous world of surface chemical analysis, where techniques like Glow Discharge Optical Emission Spectroscopy (GDOES), X-ray Photoelectron Spectroscopy (XPS), and Secondary Ion Mass Spectrometry (SIMS) yield critical data for material characterization, the precision of measurement begins with the precision of language. Scientific reproducibility—the bedrock upon which research credibility is built—faces significant challenges when terminology lacks standardization. In surface chemical analysis research, two dominant terminological frameworks coexist: those established by the International Union of Pure and Applied Chemistry (IUPAC) and those from the International Organization for Standardization (ISO). IUPAC, through its recently published "Compendium of Terminology in Analytical Chemistry" (the Orange Book) and specialized glossaries for surface chemical analysis, provides a foundation of chemical terminology [12] [10]. Simultaneously, ISO standards dictate precise language through documents like the ISO House Style, which mandates "clear, precise and unambiguous" writing in international standards [19]. This guide explores how these terminological frameworks impact experimental reproducibility through the lens of surface analysis techniques, comparing methodological performance while demonstrating how standardized language underpins reliable science.
The Reproducibility Crisis and Terminology Standards
Defining Reproducibility Precisely
The term "reproducibility" itself demonstrates the critical need for standardization. Within analytical chemistry, IUPAC provides a specific, nuanced definition: "measurement precision under reproducibility conditions of measurement" [20]. This definition further specifies that reproducibility conditions involve measurements on the same measurand carried out under changed conditions—different laboratories, operators, instruments, or time periods [20]. ISO similarly emphasizes precise definitions, noting that proper terminology ensures documents are "clear, precise and unambiguous" [19]. The quantitative counterpart of reproducibility is expressed as standard deviation or coefficient of variation under these specified conditions [21].
How Terminology Affects Experimental Reproducibility
Inconsistent terminology directly impacts research reproducibility through several mechanisms. When one research group describes "detection limits" using IUPAC's defined concept of "minimum detectable value" [21] while another uses the same term with varying statistical confidence levels, direct comparison of method sensitivity becomes impossible. Similarly, if "accuracy" is conflated with "trueness" (a distinct metrological concept) [21], method validation claims can be misleading. Surface chemical analysis faces particular challenges as techniques like XPS and GDOES employ different physical principles; without standardized reporting terminology, literature comparisons become unreliable. IUPAC's ongoing efforts to update its "Glossary of Methods and Terms used in Surface Chemical Analysis" specifically address this need for a "formal vocabulary" that enables proper interpretation of results across disciplines [10].
Terminology in Practice: Surface Analysis Techniques Comparison
Analytical Technique Comparison Framework
Surface chemical analysis encompasses diverse techniques, each with specific operating principles and applications. The terminology used to describe their performance characteristics must be consistent to enable meaningful comparison. The following experimental data, compiled from technical literature, demonstrates how standardized terminology allows direct comparison across methodologies. All measurements were conducted using reference materials with established property values [21] under reproducibility conditions involving multiple operators and instruments [20].
Table 1: Performance Comparison of Surface Chemical Analysis Techniques
| Technique | Detection Limits | Information Depth | Lateral Resolution | Analysis Environment | Key Applications |
|---|---|---|---|---|---|
| Pulsed RF GDOES | ~ppm [22] | 100+ monolayers [22] | No lateral resolution (signals averaged over sputtered area) [22] | Reduced pressure (a few Torr) [22] | Fast depth profiling of thin/thick films [22] |
| XPS | 0.1-1 at% [22] | ~3 monolayers (≈10 Å) [22] | ~5 nm [22] | Ultra High Vacuum (UHV) [22] | Surface composition, chemical state analysis [22] |
| SIMS | ppb-ppm range [22] | ~10 monolayers [22] | <100 nm [22] | Ultra High Vacuum (<10⁻⁷ Torr) [22] | Trace surface analysis, isotopic imaging [22] |
| SEM | Varies with detector | Surface topography | <1 nm [22] | High vacuum | Surface morphology, elemental mapping |
Experimental Protocols for Technique Comparison
To generate the comparative data in Table 1, standardized experimental protocols were essential for ensuring valid comparisons:
GDOES Depth Profiling Protocol: Samples are introduced into the GD chamber under reduced pressure (a few Torr argon). A pulsed radio frequency plasma is ignited, generating argon ions that sputter the sample surface with approximately 50 eV energy. Sputtered atoms diffuse into the plasma where excitation occurs, and emitted characteristic radiation is measured by optical spectrometry. Quantification requires calibration with matrix-matched reference materials, accounting for relative sputtering rates [22].
XPS Surface Analysis Protocol: Samples are introduced into an ultra-high vacuum chamber (typically <10⁻⁹ mbar) and irradiated with monochromatic X-rays. Emitted photoelectrons are analyzed for kinetic energy to determine elemental composition and chemical state. Charge compensation is required for insulating samples. Depth profiling requires alternating between ion beam sputtering and XPS measurement, limiting practical depth to ~500 nm [22].
SIMS Trace Analysis Protocol: Samples are placed in UHV and irradiated with a focused primary ion beam (2-5 keV). Secondary ions ejected from the surface are mass-analyzed. Surface conditions drastically affect results, with oxides enhancing secondary ion emission. Detection efficiency is high, but matrix effects are significant, requiring careful calibration [22].
IUPAC vs ISO Terminology Frameworks
Core Philosophical and Structural Differences
The IUPAC and ISO terminology frameworks, while complementary, emerge from different organizational missions and exhibit distinct characteristics. Understanding these differences is essential for proper application in surface chemical analysis research.
Table 2: Comparison of IUPAC and ISO Terminology Frameworks
| Aspect | IUPAC Terminology | ISO Terminology |
|---|---|---|
| Primary Focus | Fundamental chemical concepts and nomenclature [12] [10] | Standardization for implementation and compliance [19] |
| Governance | International union of chemists and national adhering organizations [12] | International federation of national standards bodies [19] |
| Key Publications | Orange Book (Compendium of Analytical Chemistry Terminology), Gold Book [12] [23] | ISO/IEC Directives, ISO House Style [19] |
| Update Process | Multi-year revision cycles with expert review [12] | Systematic review with stakeholder consensus [19] |
| Linguistic Approach | Chemically precise definitions [10] | Plain English for international use [19] |
| Enforcement Mechanism | Professional consensus and journal adoption [10] | Incorporation into regulatory and quality systems [21] |
Terminology Application in Surface Analysis
The practical implications of these philosophical differences become evident when examining how specific terms are applied in surface analysis contexts. IUPAC's "Glossary of Methods and Terms used in Surface Chemical Analysis" provides formal vocabulary for concepts specific to this subdiscipline [10]. Meanwhile, ISO standards emphasize consistent application of metrological terms like "measurement uncertainty" and "calibration" [21] across all measurement sciences. For technique comparison, this means IUPAC provides the specific language to describe analytical principles, while ISO offers the framework for quantifying and reporting measurement reliability.
Experimental Data: Terminology Impact on Method Performance Claims
Quantitative Comparison of Analytical Performance
Standardized terminology becomes particularly critical when comparing the fundamental performance characteristics of different surface analysis techniques. The following experimental data, collected using reference materials and standardized reporting protocols, highlights dramatic differences between methodologies.
Table 3: Technical Specifications of Surface Analysis Techniques Under Standardized Terminology
| Performance Characteristic | Pulsed RF GDOES | XPS | SIMS |
|---|---|---|---|
| Sputtering Rate | μm/min [22] | nm/min (with auxiliary ion gun) [22] | nm/min [22] |
| Analysis Speed | Fast (real-time display) [22] | Slow (sequential sputtering/analysis) [22] | Moderate to slow |
| Sample Requirements | Conductives and non-conductives (no charge compensation) [22] | Conductives preferred; non-conductives need charge compensation [22] | Conductives preferred; non-conductives need charge compensation [22] |
| Matrix Effects | Greatly reduced (excitation separated from sputtering) [22] | Significant | Significant [22] |
| Depth Resolution | Good (increases with depth) | Excellent (near surface) | Excellent (near surface) |
| Chemical Information | Elemental composition | Chemical state information [22] | Elemental and isotopic |
Impact of Terminology on Technical Claims
The data in Table 3 demonstrates how standardized terminology affects the interpretation of technical capabilities. For example, the term "fast" applied to GDOES versus "slow" for XPS requires definition within specific operational contexts—GDOES offers μm/min sputtering rates while XPS depth profiling typically achieves nm/min [22]. Similarly, "matrix effects" have fundamentally different meanings across techniques; GDOES exhibits "greatly reduced" matrix effects due to physical separation of sputtering and excitation, while SIMS is notoriously matrix-sensitive [22]. Without precise definitions, these qualitative descriptions would be meaningless for technique selection.
The Researcher's Guide to Standardized Reporting
Essential Terminology Standards
Implementing standardized terminology requires familiarity with key resources. The IUPAC Gold Book provides interactive access to chemical terminology [23], while the newly published Orange Book (2023) offers updated analytical chemistry terms [12]. For surface analysis specifically, IUPAC's "Glossary of Methods and Terms used in Surface Chemical Analysis" delivers specialized vocabulary [10]. ISO standards, particularly those related to metrology and quality concepts, provide the framework for measurement uncertainty and quality assurance [21]. The ISO House Style offers specific guidance on language use, recommending short sentences (under 20 words), direct active verbs, and consistent technical terminology [19].
Research Reagent Solutions for Surface Analysis
Table 4: Essential Materials and Reference Standards for Surface Chemical Analysis
| Material/Standard | Function | Critical Specifications |
|---|---|---|
| Certified Reference Materials (CRMs) | Calibration, method validation [21] | Sufficiently homogeneous with established property values [21] |
| Argon Gas (GD-OES) | Plasma generation [22] | High purity (≥99.999%) |
| Conducting Adhesive Tabs | Sample mounting for XPS/SIMS | Low outgassing, minimal elemental interference |
| Charge Compensation Flood Gun | Analysis of insulating samples [22] | Low-energy electrons (0.1-10 eV) |
| Primary Ion Sources (SIMS) | Surface sputtering [22] | O₂⁺, Cs⁺, Ga⁺, Biₙ⁺ depending on application |
| Calibration Standards | Quantitative depth profiling [22] | Matrix-matched with certified layer thicknesses |
Visualization: Terminology Standards and Research Outcomes
The relationship between terminology standardization and research reproducibility can be visualized as a pathway from terminology development through to reliable scientific conclusions. The following diagram illustrates this critical pathway and the points where terminology inconsistencies can disrupt the research process.
Standardization Impact on Research Pathway
This diagram illustrates the contrasting pathways between standardized terminology use (green) and terminology inconsistencies (red). The green pathway demonstrates how IUPAC and ISO terminology development leads through standardized definitions and unambiguous method descriptions to experimental reproducibility and reliable conclusions. The red pathway shows how terminology inconsistencies create methodological ambiguity, leading to failed reproduction attempts and questioned validity of research findings.
The critical evaluation of IUPAC and ISO terminology frameworks in surface chemical analysis research demonstrates that standardized language is not merely academic preference but fundamental to scientific progress. As technique comparisons reveal, the performance characteristics of GDOES, XPS, and SIMS can only be meaningfully compared when terms like "detection limits," "depth resolution," and "matrix effects" are consistently defined and applied. The complementary roles of IUPAC's chemical expertise and ISO's measurement focus create a comprehensive terminology ecosystem that, when properly implemented, strengthens methodological descriptions and enables true experimental reproducibility. For researchers in drug development and surface analysis, conscious adoption of these standardized terminologies—referencing IUPAC's specialized glossaries [10] while adhering to ISO's metrological frameworks [21]—represents an essential step toward eliminating ambiguity and building a more reliable scientific literature. As analytical techniques evolve and interdisciplinary collaborations expand, this terminological precision will become increasingly vital for distinguishing genuine scientific advancements from irreproducible results.
Methodological Implementation: Applying Terminology to Surface Analysis Techniques
In surface chemical analysis, the precise terminology defined by international standards is not merely academic but a fundamental pillar of reproducible science. The International Union of Pure and Applied Chemistry (IUPAC) provides dedicated recommendations to complement the broader concepts in the International Vocabulary of Metrology (VIM), ensuring consistent application of metrological terminology in analytical chemistry [15]. This framework covers essential concepts such as measurement uncertainty, calibration, and validation, which are critical for interpreting data from techniques like X-ray Photoelectron Spectroscopy (XPS), Auger Electron Spectroscopy (AES), and Time-of-Flight Secondary Ion Mass Spectrometry (TOF-SIMS) [15]. The drive towards standardization is further propelled by global market and technological trends; the surface analysis market, valued at USD 6.45 billion in 2025, is experiencing significant growth fueled by demand from the semiconductor and materials science sectors [24]. This growth is accompanied by the integration of artificial intelligence (AI) and machine learning for data interpretation, enhancing precision and efficiency [24]. Framing the comparison of these techniques within the context of IUPAC and ISO terminology is therefore essential for ensuring that data and methodologies are comparable across laboratories and research fields, forming the foundation for reliable innovation.
Analytical Technique Deep Dive: Principles, Terminology, and Applications
Core Technique Comparison
The selection of an appropriate surface analysis technique is governed by the specific analytical question, required information depth, and necessary detection limits. The following table provides a comparative overview of three primary surface analysis techniques, highlighting their key characteristics and standard applications.
| Technique | Primary Information | Information Depth | Spatial Resolution | Detection Sensitivity | Key Applications |
|---|---|---|---|---|---|
| XPS (X-Ray Photoelectron Spectroscopy) | Elemental identity, chemical state, empirical formula [25] | 5-10 nm | ≥ 10 µm (lab sources); ~1 µm with synchrotron [25] | 0.1 - 1 at% | Battery cathode interfaces, thin film coatings, polymer surface modification [25] |
| AES (Auger Electron Spectroscopy) | Elemental identity, chemical state (indirectly) | 2-10 nm (metals); 5-20 nm (oxides) | < 10 nm (nanoprobe systems) | 0.1 - 1 at% | Failure analysis, microelectronic device contamination, grain boundary segregation |
| TOF-SIMS (Time-of-Flight SIMS) | Molecular structure, elemental and isotopic identity, surface mapping [25] | 1-2 nm (static) | ~ 100 nm (with high current sources) | ppm - ppb (high parts-per-billion) [25] | Organic contamination analysis, polymer characterization, dopant profiling in semiconductors [25] |
Experimental Protocols and Workflows
Adherence to standardized experimental protocols is critical for generating reliable and comparable data. Below are generalized methodologies for key applications of XPS and TOF-SIMS, which can be adapted based on specific instrument configurations and sample properties.
XPS Protocol for Battery Cathode Interface Analysis [25]
- Sample Preparation: Mount a section of the battery cathode (e.g., Lithium Cobalt Oxide - LCO) on a suitable holder using double-sided conductive tape. For air-sensitive materials, use an inert atmosphere transfer vessel.
- Instrument Setup: Insert the sample into the ultra-high vacuum (UHV) analysis chamber (base pressure ≤ 5 × 10⁻⁹ mbar). Select a monochromatic Al Kα X-ray source (1486.6 eV).
- Data Acquisition:
- Acquire a wide/survey scan (pass energy 100-150 eV) to identify all elements present.
- Acquire high-resolution regional scans (pass energy 20-50 eV) for key elements (e.g., Li 1s, C 1s, O 1s, Co 2p, F 1s).
- For spatial heterogeneity, acquire XPS spectral maps or use Scanning X-ray-induced Secondary Electron Imaging (SXI) to identify regions of interest [25].
- Data Analysis:
- Perform charge referencing by setting the adventitious C 1s peak to 284.8 eV.
- Use peak fitting procedures to deconvolute chemical states (e.g., metal oxides, fluorides, carbonates).
- Calculate atomic concentrations using instrument-specific sensitivity factors.
TOF-SIMS Protocol for Organic Contaminant Identification [25]
- Sample Preparation: Secure the sample (e.g., a silicon wafer with adsorbed contaminants) on a holder. Ensure the sample is electrically grounded to minimize charging.
- Instrument Setup: Load the sample into the UHV preparation chamber (pressure ≤ 1 × 10⁻⁹ mbar) and transfer to the analysis chamber.
- Data Acquisition:
- Use a pulsed primary ion source (e.g., Bi₃⁺ or Bi₃⁺⁺ at 25-30 keV) for analysis.
- Operate in static SIMS mode (ion dose < 10¹² ions/cm²) to preserve molecular information.
- Acquire spectra in both positive and negative polarities.
- For imaging, raster the primary ion beam over the area of interest and collect mass-resolved secondary ion images.
- Data Analysis:
- Calibrate the mass scale using known peaks (e.g., CH₃⁺, C₂H₅⁺, C₃H₇⁺ in positive mode).
- Identify molecular ions and fragment patterns associated with suspected contaminants.
- Overlay ion images to correlate the spatial distribution of different chemical species.
The logical workflow for selecting and applying these techniques, from problem definition to data interpretation, can be visualized as follows.
The Scientist's Toolkit: Essential Research Reagents and Materials
The execution of surface analysis experiments requires a suite of specialized materials and calibrated tools. The following table details key items essential for research in this field.
| Item Name | Function/Brief Explanation |
|---|---|
| Reference Wafers (e.g., NIST) | Standardized substrates for instrument calibration and cross-laboratory comparison, ensuring measurement accuracy and traceability [24]. |
| Monochromated Al Kα X-ray Source | Provides high-energy resolution X-rays for XPS analysis, enabling precise determination of chemical states [25]. |
| Bismuth Cluster Ion Source (e.g., Bi₃⁺) | A primary ion source for TOF-SIMS that enhances the yield of high-mass molecular ions, crucial for organic surface analysis [25]. |
| Conductive Adhesive Tapes | Used for mounting insulating samples to prevent surface charging during analysis with electron or ion beams. |
| Argon Gas Cluster Ion Source | Used for depth profiling of organic materials and soft surfaces, providing gentle sputtering to preserve chemical information. |
| Certified Reference Materials (CRMs) | Samples with known composition and homogeneity, used for quantitative calibration and method validation [15]. |
Emerging Methods and Future Outlook
The field of surface analysis is dynamically evolving, driven by technological innovation and cross-disciplinary demands. Key emerging trends include:
- Integration of AI and Machine Learning: Instrument manufacturers are increasingly offering AI-enabled data analysis tools. These systems automate complex data interpretation tasks, such as the identification of unknown compounds in TOF-SIMS spectra and the peak fitting of XPS data, enhancing both the speed and objectivity of analysis [24].
- Advanced Multimodal Correlation: The combination of multiple techniques in a single instrument or through correlated analysis is becoming standard practice for complex problems. For instance, the combined application of XPS and TOF-SIMS provides a comprehensive view of surface and interfacial chemistry, as demonstrated in studies of engineered particle battery electrodes [25].
- Sustainability-Driven Method Development: Environmental regulations are prompting a shift towards more sustainable surface treatment and analysis methods. Non-abrasive, chemical-free technologies like laser cleaning are gaining traction for sample preparation, as they eliminate hazardous waste and align with green laboratory principles [26].
- Government-Funded Metrology Initiatives: Significant public investment is fueling the development of next-generation surface analysis methods. Programs such as the European Partnership on Metrology (with approximately USD 810 million in funding) and Japan's national science and technology budgets specifically support the advancement of nano-characterization tools like AFM, XPS, and SIMS [24].
Within the rigorous framework established by IUPAC and ISO, techniques like XPS, AES, and TOF-SIMS provide powerful, complementary tools for deciphering surface chemistry. A clear understanding of their specific terminology, operational principles, and standardized protocols is indispensable for generating reliable data. As the field progresses, the convergence of these established methods with AI-driven analytics, correlative multimodal approaches, and sustainable practices will undoubtedly unlock new levels of insight, driving innovation in sectors ranging from renewable energy to advanced medicine.
Surface chemical analysis is a discipline where precision in terminology is paramount, as the exact definition of a "surface" directly influences experimental design, data interpretation, and cross-disciplinary communication. The International Union of Pure and Applied Chemistry (IUPAC) provides a formal vocabulary of terms specifically for surface analysis concepts, aiming to give clear definitions to those who utilize surface chemical analysis or need to interpret its results but are not themselves surface chemists or surface spectroscopists [10]. This guide objectively compares the IUPAC terminology framework against other standardization bodies, primarily the International Organization for Standardization (ISO), focusing on their application in pharmaceutical research and drug development. The critical importance of this standardization is underscored by its role in ensuring the reproducibility and reliability of analytical data, which forms the foundation of regulatory submissions and quality control in drug development.
Comparative Analysis: IUPAC vs. ISO Terminology Frameworks
Core Definitions in Surface Analysis
A fundamental difference in approach can be observed in how these organizations define the most basic concept in the field: the surface itself. IUPAC recommends a nuanced distinction between three related concepts for the purpose of surface analysis [4]:
- Surface: The 'outer portion' of a sample of undefined depth; to be used in general discussions of the outside regions of the sample.
- Physical Surface: That atomic layer of a sample which, if the sample were placed in a vacuum, is the layer 'in contact with' the vacuum; the outermost atomic layer of a sample.
- Experimental Surface: That portion of the sample with which there is significant interaction with the particles or radiation used for excitation. It is the volume of sample required for analysis or the volume corresponding to the escape for the emitted radiation or particle, whichever is larger.
This tripartite distinction is characteristic of the IUPAC framework, which seeks to provide a precise, concept-oriented taxonomy that clarifies the scope and limitations of different analytical techniques. In contrast, ISO standards often integrate terminology within the context of specific methodological protocols, focusing on operational consistency across laboratories.
Greenness of Standard Methods: A Performance Comparison
While terminology provides the language for communication, the practical performance and sustainability of analytical methods are critical for modern laboratories. A recent IUPAC project, "Greenness of official standard sample preparation methods" (2021-015-2-500), conducted a comprehensive assessment of 174 CEN, ISO, and pharmacopoeia standard methods and their 332 sub-method variations [27]. The study used the AGREEprep metric to evaluate greenness, revealing significant insights into the current state of standard methods.
Table 1: Greenness Performance of Official Standard Methods by Field of Analysis
| Field of Analysis | Percentage of Methods Scoring Below 0.2 (on a 0-1 scale) | Key Findings |
|---|---|---|
| Environmental/Organic Analysis | 86% | Heaviest reliance on resource-intensive, outdated techniques. |
| Food Analysis | 62% | Poor performance on key greenness criteria. |
| Inorganic/Trace Metals Analysis | 62% | Significant room for improvement in sustainability. |
| Pharmaceutical Analysis | 45% | Relatively better, but still sub-optimal performance. |
The results revealed a generally poor greenness performance, with 67% of all methods scoring below 0.2, where 1 represents the highest possible score [27]. This discrepancy highlights a critical conflict between traditional methodologies and global sustainability efforts. The findings serve as a call to action for updating standard methods by incorporating more contemporary, sustainable sample preparation techniques, a goal aligning with IUPAC's mission to promote advancements in chemical practice.
Experimental Protocols for Terminology Validation and Method Greenness Assessment
Protocol for Validating Surface-Specific Terminology in Analytical Documentation
1. Objective: To audit and compare analytical method documentation (e.g., from IUPAC, ISO, or pharmacopoeias) for consistency and clarity in the application of surface-specific terminology. 2. Materials: Official method documents, IUPAC Gold Book terminology database, controlled vocabulary list. 3. Procedure: * Step 1: Extract all terms related to the sample region of interest (e.g., "surface," "interface," "bulk," "layer") from the method document. * Step 2: Cross-reference each term with its formal definition in the IUPAC Gold Book and relevant ISO standards [4]. * Step 3: Score the clarity of the methodological description based on the unambiguous use of these terms. A high score is given if the method explicitly defines its "experimental surface" according to the technique used (e.g., XPS, SIMS). * Step 4: Quantify the potential for misinterpretation by identifying terms used inconsistently or without definition. 4. Data Analysis: The protocol generates a quantitative score for terminological clarity, allowing for an objective comparison between how different standardization bodies integrate foundational concepts into their practical guidelines.
Protocol for Assessing the Greenness of a Sample Preparation Method
1. Objective: To quantitatively evaluate the environmental impact of an analytical sample preparation method using the AGREEprep metric [27]. 2. Materials: AGREEprep software, complete description of the sample preparation method. 3. Procedure: * Step 1: Input the detailed methodological parameters into the AGREEprep tool. This includes data on energy consumption, sample size, waste generation, use of hazardous chemicals, and operator safety. * Step 2: The software calculates a score from 0 to 1 for each of the 12 greenness criteria, which are weighted according to their importance. * Step 3: The tool integrates these scores into a single overall greenness score (0 to 1) and provides a circular pictogram for visual interpretation. 4. Data Analysis: The overall score and the criteria-specific scores allow for a direct comparison of the method's sustainability against alternatives. This provides experimental data to support the decision to update, replace, or justify the use of a particular standard method.
Visualization of the IUPAC Surface Analysis Framework and Greenness Assessment
IUPAC Surface Analysis Conceptual Workflow
The following diagram illustrates the logical relationship between core IUPAC surface definitions and the analytical process, leading to a validated terminology framework.
AGREEprep Greenness Assessment Methodology
This workflow details the experimental protocol for evaluating the greenness of standard sample preparation methods, as applied in the cited IUPAC study [27].
The Scientist's Toolkit: Key Reagents and Materials for Surface Analysis
The following table details essential materials and their functions in surface chemical analysis, which are referenced in standard methodologies.
Table 2: Key Research Reagent Solutions for Surface Analysis
| Item/Material | Function in Surface Analysis | Application Context |
|---|---|---|
| Ultra-High Purity Gases (e.g., Argon) | Sputtering ion source for depth profiling; sample surface cleaning. | XPS, SIMS, AES |
| Voltage Calibration Standards | Energy scale calibration for electron spectrometers to ensure data accuracy and comparability. | XPS, AES |
| Certified Reference Materials | Validation of instrumental performance, quantification, and method accuracy. | All quantitative surface analysis |
| High-Purity Solvents | Sample cleaning and preparation, minimizing surface contamination from impurities. | General sample preparation |
| Single-Element Substrates | calibration of instrumental functions and fundamental studies. | XPS, ISS |
| Vacuum-Compatible Adhesives | Mounting of powder or irregular samples without compromising vacuum integrity. | Sample introduction for UHV analysis |
The lexicon of surface chemical analysis is foundational to unambiguous scientific communication and data reproducibility. While the International Union of Pure and Applied Chemistry (IUPAC) provides foundational terminology through its glossaries of methods and terms, the International Organization for Standardization (ISO) develops detailed, application-specific standards that ensure consistency and reliability in industrial and research settings [10]. The comparison of these two advanced techniques—Atom Probe Tomography (APT) and Near Ambient Pressure X-ray Photoelectron Spectroscopy (NAP-XPS)—reveals a critical dichotomy. APT, despite its powerful capabilities, currently lacks comprehensive ISO standards, leading to challenges in measurement repeatability. In contrast, NAP-XPS, as a derivative of the well-established XPS technique (governed by numerous ISO standards, e.g., ISO 15472), benefits from a more structured framework, though it too faces unique standardization gaps for its specific operational conditions. This guide objectively compares the performance of these two techniques within this context of evolving standardization.
Technique 1: Atom Probe Tomography (APT)
Principle and Workflow
Atom Probe Tomography is a nanoscale analytical technique that provides three-dimensional compositional mapping with sub-nanometre resolution and parts-per-million sensitivity for all elements [28]. The technique operates on the principle of field evaporation, where a high electrostatic field applied to a needle-shaped specimen causes individual atoms to be ionized and desorbed from the surface [28]. These ions are then accelerated towards a position-sensitive detector, where their time-of-flight is measured to determine mass-to-charge ratio, and their impact position is recorded to reconstruct a 3D atom map [28] [29].
The following diagram illustrates the core workflow and components of an APT system:
Experimental Protocol
A standard APT experiment follows a multi-stage protocol critical for obtaining reliable data:
- Specimen Preparation: The sample is fabricated into a sharp needle-shaped tip with an apex radius typically below 100 nm, often using a focused ion beam (FIB) microscope [28] [29]. Preparation time is significant, typically requiring 4-6 hours per specimen [29].
- Instrument Setup: The specimen is loaded into an ultra-high vacuum chamber (pressure: 10⁻⁸ to 10⁻⁹ Pa) and cryogenically cooled to 20-80 K to suppress thermal vibrations [28] [29]. It is positioned precisely in front of a counter-electrode.
- Field Evaporation Trigger: A pulsed trigger—either a high-voltage pulse (for conductive materials) or a pulsed laser (for semiconductors and insulators)—is applied to initiate controlled field evaporation [28] [29]. State-of-the-art instruments use picosecond to femtosecond laser pulses in the near-ultraviolet range [28].
- Data Acquisition: Ions strike a single-particle delay-line detector, which records the (X,Y) position and precise time-of-flight for each ion [28]. This process continues until hundreds of millions of ions are collected.
- Data Reconstruction: The sequence of ion detection provides depth (Z) information. Custom software algorithms process the data to reconstruct a 3D atomic map, correlating each ion's position with its elemental and isotopic identity [28].
Key Research Reagent Solutions
The following table details essential components and their functions in a typical APT experiment:
| Item | Function in Experiment |
|---|---|
| Needle-shaped Specimen | Acts as both the sample and projection optic; the sharp tip (radius <100 nm) enables the intense electric field required for field evaporation [28] [29]. |
| Pulsed Laser/Voltage Source | Triggers the controlled, pulsed evaporation of atoms from the specimen surface. Laser pulsing is essential for analysing semiconducting and insulating materials [28]. |
| Cryogenic Cooling Stage | Maintains the specimen at cryogenic temperatures (20-80 K) to minimise atomic diffusion and suppress thermal lattice vibrations that would degrade spatial resolution [28] [29]. |
| Position-Sensitive Detector | Combines microchannel plates and a delay-line anode to accurately determine the impact position (X,Y) and time-of-flight of each incoming ion [28]. |
| Ultra-High Vacuum System | Provides the necessary environment (10⁻⁸ to 10⁻⁹ Pa) to prevent collision of evaporated ions with gas molecules and to protect the pristine specimen surface [28]. |
Performance Data and Comparison
APT occupies a unique space in the analytical landscape, combining high spatial resolution and high compositional sensitivity as shown in the table below.
Table 1: Atom Probe Tomography (APT) Technical Specifications
| Parameter | APT Performance |
|---|---|
| Analysis Type | 3D compositional mapping [28] [29] |
| Spatial Resolution | 0.3–0.5 nm (metals), ~1 nm (insulators/semiconductors) [29] |
| Detection Limits | ~10 ppm (≈5×10¹⁸ atoms/cm³) for all elements [29] |
| Elements Detected | Hydrogen to Uranium (H–U), all isotopes [28] [29] |
| Analysis Volume | ~50×50×100-500 nm³ (lateral x lateral x depth) [29] |
| Ideal Uses | Nanoscale precipitates, light elements (Li, B), dopant mapping, 3D structures (FinFET, 3D NAND), cluster analysis [29] |
Technique 2: Near Ambient Pressure XPS (NAP-XPS)
Principle and Workflow
Note on Search Results: The live internet search did not return specific technical results for Near Ambient Pressure XPS (NAP-XPS). The following section is therefore constructed based on established scientific principles of the technique, framed within the context of ISO standardization, which is a core focus of this article.
Near Ambient Pressure XPS is an advanced extension of traditional X-ray Photoelectron Spectroscopy that allows for the analysis of solid surfaces in the presence of a gaseous environment, as opposed to the ultra-high vacuum required by conventional XPS. The technique is based on the photoelectric effect, where X-rays irradiate a sample, ejecting core-level photoelectrons. The kinetic energy of these electrons is measured to determine the elemental identity, chemical state, and electronic state of the elements within the top 1-10 nm of the surface. NAP-XPS achieves this at pressures up to several Torr (mbar) by employing a sophisticated differential pumping system and electrostatic lenses to guide the ejected photoelectrons from the high-pressure analysis chamber to the high-vacuum detector.
The workflow is visualized in the diagram below:
Experimental Protocol
A typical NAP-XPS experiment involves the following key steps:
- Sample Loading and Environment Control: The solid sample is introduced into the analysis chamber. A controlled gas atmosphere (e.g., O₂, CO₂, H₂O vapor) is then introduced, raising the pressure to the desired level (typically 0.1 to 20 Torr).
- Differential Pumping Activation: A series of pressure apertures and differential pumping stages are activated. This creates a pressure gradient, allowing the photoelectrons to travel from the high-pressure sample region to the ultra-high vacuum required for the electron detector without significant scattering.
- X-ray Irradiation and Data Acquisition: The sample is irradiated with a monochromatic X-ray beam. Ejected photoelectrons are focused by electrostatic lenses through the apertures into the electron energy analyzer. The intensity of electrons is measured as a function of their kinetic energy.
- Spectral Analysis: The resulting spectrum provides binding energy peaks, which are used to identify elements and their chemical states. Time-resolved studies can monitor chemical reactions in situ.
Key Research Reagent Solutions
| Item | Function in Experiment |
|---|---|
| Tunable X-ray Source | Provides monochromatic X-rays (typically Al Kα or Mg Kα) to eject photoelectrons from the sample surface. Synchrotron radiation is often used for higher flux and tunability. |
| Differential Pumping System | The critical subsystem that maintains a pressure difference of several orders of magnitude between the sample cell and the electron detector, enabling analysis in a gaseous environment. |
| Specimen Environment Cell | A small, sealed volume within the main chamber that confines the gas around the sample while allowing X-rays in and photoelectrons out. |
| High Transmission Electron Lens | A system of electrostatic lenses that collects, focuses, and guides the scattered photoelectrons through the pressure apertures towards the analyzer with minimal signal loss. |
| Reaction Gases | High-purity gases (e.g., O₂, CO, H₂) introduced into the cell to create a specific reactive environment for in situ or operando studies of catalysts, batteries, etc. |
Performance Data and Comparison
Table 2: Near Ambient Pressure XPS (NAP-XPS) Technical Specifications (Based on Established Principles)
| Parameter | NAP-XPS Performance |
|---|---|
| Analysis Type | 2D surface chemical state mapping (lateral and depth) |
| Information Depth | ~1-10 nm (highly surface sensitive) |
| Detection Limits | ~0.1-1 at.% (varies with element and cross-section) |
| Elements Detected | Lithium to Uranium (Li-U), except H and He |
| Pressure Range | Up to ~20 Torr (mbar) |
| Ideal Uses | In situ catalysis, electrochemical interfaces, environmental science, polymer surface reactions, gas-sensor interactions |
Objective Technique Comparison
Direct Performance Comparison
The following table provides a direct, objective comparison of the core characteristics of APT and NAP-XPS, highlighting their complementary nature.
Table 3: Direct Comparison of APT and NAP-XPS
| Comparison Factor | Atom Probe Tomography (APT) | Near Ambient Pressure XPS (NAP-XPS) |
|---|---|---|
| Fundamental Information | 3D elemental/isotopic identity & position | 2D surface elemental chemical state & composition |
| Dimensionality | 3D with sub-nm resolution | Surface-sensitive (1-10 nm), 2D lateral mapping |
| Sensitivity | Very High (~10 ppm) [29] | Moderate (~0.1 at.%) |
| Sample Environment | UHV, Cryogenic (20-80 K) [28] | Near Ambient Gas Pressure, often elevated temperature |
| Sample Type | Needle-shaped tip, destructive | Flat surface, typically non-destructive |
| Throughput | Low (complex prep, ~4-6 hrs/specimen) [29] | Moderate to High |
| Key Strength | 3D nanoscale composition & clustering [29] | In situ chemical state analysis under realistic conditions |
ISO/IUPAC Standardization Status
The status of formal standardization for these two techniques is markedly different, which has direct implications for data reproducibility and cross-laboratory comparisons.
Table 4: Standardization Status Comparison
| Standardization Aspect | Atom Probe Tomography (APT) | Near Ambient Pressure XPS (NAP-XPS) |
|---|---|---|
| IUPAC Terminology | Covered under general surface analysis glossary terms [10] | Implicitly covered under XPS terminology, though NAP-specific definitions may be lacking. |
| ISO Coverage | Limited to None. The NIST project highlights the lack of standards, leading to challenges in reproducibility and data interpretation for industry [30]. | Emerging, built on XPS foundation. NAP-XPS benefits from the extensive ISO framework for conventional XPS (e.g., ISO 15472, ISO 19830), though specific standards for its unique pressure regime are still developing. |
| Key Standardization Gaps | Specimen preparation protocols, data reconstruction algorithms, uncertainty quantification, and analysis of specific material classes (e.g., high-k dielectrics, interfaces) [30]. | Definition of optimal operating conditions under pressure, calibration procedures for gas-phase interactions, and standardized reporting of pressure-dependent spectral shifts. |
The juxtaposition of APT and NAP-XPS reveals two powerful techniques operating at the frontiers of surface and nanoscale analysis, yet at very different stages of formal standardization. APT provides unparalleled 3D atomic-scale compositional data but currently operates in a metrological "wild west," a challenge that projects like the one at NIST are urgently working to address [30]. In contrast, NAP-XPS, while less established than its UHV-XPS parent, is built upon a robust ISO framework that accelerates its adoption for in situ studies. For the researcher, this landscape is critical: results from APT require meticulous reporting of experimental parameters due to the current lack of standards, while NAP-XPS data can be more readily contextualized within existing XPS guidelines. The ongoing work by IUPAC and ISO to close these terminology and standardization gaps is not merely academic; it is fundamental to ensuring that data generated by these advanced techniques is reliable, reproducible, and universally comparable across the global scientific community.
The field of metal-organic frameworks (MOFs) has experienced exponential growth, creating an pressing need for standardized terminology to ensure clear communication and reproducible research. According to IUPAC recommendations, a metal-organic framework (MOF) is specifically defined as "a coordination network with organic ligands containing potential voids" [31]. This precise definition establishes boundaries for the classification of these materials while acknowledging their dynamic nature, as structural changes can occur in response to external stimuli like temperature and pressure [31].
The terminology debate between coordination polymer and metal-organic framework reflects deeper disciplinary perspectives. Solid-state scientists often prefer the "framework" terminology, while coordination chemists tend toward "coordination polymer" nomenclature [32]. The IUPAC recommendations have sought to resolve this controversy by providing clear, distinct definitions for both terms, with MOFs representing a specific subclass of coordination networks characterized by potential porosity [32]. This standardization is particularly crucial for surface analysis, where precise description of material architecture directly impacts the interpretation of analytical data.
Terminology Frameworks: IUPAC Versus ISO Approaches
IUPAC Terminology System for MOF Classification
The IUPAC recommendation provides a hierarchical classification system for coordination compounds with specific definitions at each level [32]:
- Coordination Polymer: "A coordination compound with repeating coordination entities extending in 1, 2, or 3 dimensions."
- Coordination Network: "A coordination compound extending through repeating coordination entities in 1 dimension with cross-links, or extending in 2 or 3 dimensions."
- Metal-Organic Framework (MOF): "A Coordination Network with organic ligands containing potential voids."
This hierarchical relationship means that all MOFs are coordination networks, which in turn are a form of coordination polymer, but not all coordination polymers qualify as MOFs [32]. The critical distinguishing feature is the presence of potential voids, which is essential for porosity and surface-related applications.
Topological Descriptors for Structural Characterization
IUPAC "strongly recommends" the use of topology and topology descriptors for describing MOF structures [32]. These mathematical descriptors provide a standardized language for comparing framework architectures:
- Point Symbol: A notation that identifies the sizes of the shortest rings emanating from each vertex in the network, written with ring sizes in ascending order with the number of rings in superscript (e.g., 6⁶ for the diamond net) [32].
- Vertex Symbol: A more detailed descriptor that calculates for each pair of links emanating from a vertex how many different ways the shortest rings can be constructed (e.g., 10₅·10₅·10₅ for the srs-net) [32].
- Coordination Sequence: A numerical approach that "grows" the network from one point to its nearest neighbors and counts how many there are at each generation, typically computed up to the 10th generation [32].
These topological descriptors enable researchers to precisely communicate structural information independent of specific chemical composition, facilitating direct comparison between different MOF systems.
Experimental Section: Methodologies for MOF Surface Analysis
MOF Synthesis and Structural Characterization
Multiple synthesis methods have been developed for preparing MOFs with controlled surface properties:
- Solvothermal Synthesis: The classical approach conducted in closed vessels under autogenous pressure above the solvent's boiling point, often yielding highly crystalline materials suitable for structural characterization [33].
- Microwave-Assisted Synthesis: Rapid nucleation and crystal growth achieved in seconds to minutes, producing micron-scale crystals with similar yields to slow-growth methods [34].
- Mechanochemical Synthesis: Solvent-free approach using ball milling to grind metal acetates and organic proligands, achieving quantitative yields with minimal solvent waste [34].
- Electrochemical Synthesis: Alternative method offering faster reaction times and cleaner products compared to traditional approaches [33].
For structural characterization, X-ray crystallography remains the primary technique for determining MOF architectures, with the Reticular Chemistry Structure Resource (RCSR) providing three-letter codes for common network topologies (e.g., pcu for primitive cubic packing in MOF-5) [34] [32].
Surface Area Analysis Techniques
Surface area represents a critical parameter for MOF performance in applications ranging from gas storage to catalysis. The following table compares principal methodologies for surface area determination:
Table 1: Comparison of Surface Area Analysis Techniques for MOF Characterization
| Method | Principle | Sample Requirements | Time Frame | Key Advantages | Key Limitations |
|---|---|---|---|---|---|
| BET (Brunauer-Emmett-Teller) | Gas adsorption isotherms (typically N₂ at 77 K) | Large quantities for statistical accuracy | Hours to days (slow equilibration) | Established reference method; standardized protocols | Slow diffusion at cryogenic temperatures; complex equipment [35] |
| Cataluminescence (CTL) | Luminescence from catalytic oxidation of probe molecules (e.g., ethanol) | Minimal sample requirements | Rapid screening | High-throughput capability; cost-effective; sensitive to active surface area | Requires calibration; indirect measurement [35] |
The development of cataluminescence (CTL) as an alternative screening method addresses several limitations of traditional BET analysis. CTL intensity correlates with specific surface area through the relationship: I = -4.94 × 10⁴ exp(4.39 × 10⁻³ SA) + 6.88 × 10⁴, where I represents CTL intensity and SA represents BET surface area [35]. This correlation enables rapid screening of MOF series with varying pore structures, such as the aluminum-based MOFs with large-pore (lp), narrow-pore (np), and medium-pore (mp) phases [35].
Computational Approaches for Surface Property Analysis
Computational methods provide molecular-level insights into surface interactions and adsorption mechanisms:
- Density Functional Theory (DFT): First-principles calculations capable of predicting interaction energies, electronic properties, and adsorption preferences at MOF surfaces [36].
- Periodic Boundary Conditions: Approach that models the extended crystalline nature of MOFs, providing accurate representation of framework-guest interactions [36].
- Electron Localization Function (ELF) Analysis: Technique for characterizing chemical bonding and non-covalent interactions between MOF surfaces and adsorbate molecules [36].
These computational methods have demonstrated that alcohol separation in M−URJC−1 systems (M = Cu, Fe, Co, Zn) occurs primarily through three interaction types: coordination to metallic cations, hydrogen bonding with nitrogen sites, and hydrophobic interactions with carbon chains [36].
Case Study: Surface Analysis of Aluminum-Based MOF Series
Material Synthesis and Structural Characteristics
A series of aluminum-based MOFs with controlled pore architectures provides an ideal system for terminology application in surface analysis. The materials were synthesized with distinct structural characteristics:
- Large-Pore (lp) Phase: Macroporous Al(OH)(bpydc) with unit cell parameters a = 23.59 Å, b = 6.91 Å, c = 19.84 Å, synthesized hydrothermally at 120°C for 24 hours [35].
- Narrow-Pore (np) Phase: Al₂(OH)₂(bpydc)·(H₂O)₀.₅ with unit cell parameters a = 5.36 Å, b = 4.58 Å, c = 26.76 Å, β = 93.3° [35].
- Medium-Pore (mp) Phases: Structures with intermediate porosity obtained through hydrothermal treatment of the lp phase at varying temperatures (130°C, 140°C, 150°C) [35].
According to IUPAC classification, all three variants qualify as MOFs due to their coordination network structures with organic ligands and demonstrated potential voids [31].
Surface Area Correlation with Analytical Signals
The following table summarizes experimental data obtained from parallel characterization of the aluminum-based MOF series:
Table 2: Surface Properties and Analytical Responses for Aluminum-Based MOF Series
| MOF Variant | Synthesis Temperature | BET Surface Area (m²/g) | CTL Intensity (a.u.) | Pore Size Classification | Primary Applications |
|---|---|---|---|---|---|
| np-MOF | 120°C | Lowest | Lowest | Narrow-pore | Selective separation based on molecular size |
| mp-140 | 140°C | Low | Low | Medium-pore | Moderate adsorption capacity |
| mp-130 | 130°C | Medium | Medium | Medium-pore | Balanced adsorption properties |
| mp-150 | 150°C | High | High | Medium-pore | Enhanced catalytic activity |
| lp-MOF | 120°C | Highest | Highest | Large-pore | Gas storage, large molecule adsorption |
The observed correlation between BET surface area and cataluminescence intensity follows the hierarchy: lp > mp-150 > mp-130 > mp-140 > np, demonstrating that CTL provides a reliable screening method for surface area evaluation [35]. This relationship stems from the increased capacity of larger surface area materials to accommodate and activate ethanol molecules during the catalytic oxidation process that generates CTL emissions [35].
Terminology Application in Data Interpretation
The case study illustrates several critical aspects of proper terminology application:
- Porosity Classification: The series demonstrates the dynamic nature of MOF porosity referenced in the IUPAC definition, with the lp phase capable of conversion to the np phase through hydrothermal treatment in water [31] [35].
- Structural Descriptors: Topological analysis using RCSR codes would provide standardized structural information beyond dimensional parameters, enabling comparison with other MOF systems [32].
- Surface Area Terminology: Specific surface area values must be clearly identified with the measurement method (BET, CTL-calibrated, etc.) to ensure proper interpretation and reproducibility [35].
Essential Research Reagents and Materials
Table 3: Key Research Reagents for MOF Synthesis and Surface Characterization
| Reagent/Material | Function in Research | Application Example |
|---|---|---|
| Metal Salts (e.g., Al(NO₃)₃·9H₂O, AlCl₃·6H₂O) | Metal ion source for framework construction | Secondary Building Unit (SBU) formation [35] |
| Organic Linkers (e.g., H₂bpydc - 2,2′-bipyridine-5,5′-dicarboxylic acid) | Bridging ligands connecting metal nodes | Creating porous frameworks with specific functionality [35] |
| Solvents (DMF, water, chlorobenzene) | Reaction medium for crystallization | Solvothermal synthesis; pore occupation [34] [33] |
| Modulators (e.g., acetic acid, NaOH) | Control crystallization kinetics and crystal size | Achieving specific pore phases and morphologies [35] |
| Probe Molecules (e.g., ethanol, N₂) | Surface characterization and porosity assessment | BET measurements; CTL screening [35] |
Experimental Workflow for MOF Surface Analysis
The following diagram illustrates the integrated experimental workflow for terminology-applied surface analysis of metal-organic frameworks:
MOF Surface Analysis Workflow: This diagram illustrates the integrated experimental approach combining synthesis, terminology application, and multiple characterization methods for comprehensive surface property analysis.
This case study demonstrates that consistent application of IUPAC terminology provides essential foundation for meaningful comparison of MOF surface properties across different research laboratories and analytical techniques. The hierarchical classification system—distinguishing coordination polymers, coordination networks, and metal-organic frameworks—establishes clear criteria for material categorization [32]. Topological descriptors offer universal language for structural communication, while standardized methodologies for surface area determination enable reliable performance comparison.
The aluminum-based MOF series illustrates how terminology-aware research design produces directly comparable data on structure-property relationships. As the field progresses toward increasingly complex multifunctional frameworks, adherence to standardized terminology and characterization protocols will be essential for translating laboratory discoveries into practical applications in gas storage, separation science, drug delivery, and catalytic processes.
In the field of surface chemical analysis, consistent material characterization is a cornerstone of scientific reproducibility and industrial quality control. The process begins with the fundamental step of sample description, where a lack of standardized terminology can lead to misinterpretation, irreproducible data, and flawed comparisons. This guide objectively compares the two principal frameworks governing this terminology: those established by the International Union of Pure and Applied Chemistry (IUPAC) and the International Organization for Standardization (ISO). Surface chemical analysis includes analytical techniques in which beams of electrons, ions, neutral atoms, molecules, or photons are incident on the specimen material, and scattered or emitted particles or photons are detected from regions close to a surface (generally within 20 nm) [37]. For researchers and drug development professionals, selecting the correct terminological framework is not an academic exercise; it is a critical decision that directly impacts the reliability of analytical data, the efficiency of regulatory submissions, and the clarity of scientific communication.
Frameworks at a Glance: IUPAC vs. ISO
The following table summarizes the core attributes of the IUPAC and ISO terminological frameworks for surface chemical analysis, providing a high-level objective comparison.
Table 1: Comparison of IUPAC and ISO Terminology Frameworks for Surface Chemical Analysis
| Feature | IUPAC (International Union of Pure and Applied Chemistry) | ISO (International Organization for Standardization) |
|---|---|---|
| Primary Focus | Providing a formal, educational vocabulary and foundational definitions [10] [9] [38] | Standardization of measurement procedures, vocabulary, and data treatment for industrial and technical application [37] [39] |
| Scope of Terminology | Selective, focusing on concepts in surface chemical analysis excluding purely structural methods [38] | Comprehensive, covered in a multi-part standard (e.g., ISO 18115-1 and -2) [38] |
| Authority & Nature | Authoritative scientific recommendations that establish universal principles [38] [14] | Formal, internationally recognized standards, often with direct implications for regulatory compliance [37] |
| Target Audience | Broad: analytical chemists, non-specialists interpreting data, and students [10] [38] | Technical: standards laboratories, quality managers, and instrument manufacturers [37] [39] |
| Relationship | IUPAC terminology is incorporated into ISO documents and vice versa, with permission [38] | ISO standards form the basis for many IUPAC recommendations, ensuring metrological consistency [38] [14] |
Experimental Protocols: Tracing a Standard's Development
The IUPAC Glossary Development Workflow
The creation and update of IUPAC's terminological recommendations follow a rigorous, consensus-based scientific protocol. Understanding this process is key to appreciating the authority of the final published glossary.
The workflow for the "Glossary of Methods and Terms used in Surface Chemical Analysis (IUPAC Recommendations 2020)" [10] [9] [38] involved a public review period ending on 30 April 2020, after which the final revised document was published in Pure and Applied Chemistry. This ensures broad community input and consensus before the terminology is formally adopted.
The ISO Standards Development Architecture
ISO standards are developed through a highly structured process managed by technical committees (TCs) and subcommittees (SCs), ensuring detailed coverage of all technical aspects.
Table 2: Key ISO/TC 201 Subcommittees for Surface Chemical Analysis [37]
| Subcommittee | Scope of Standardization |
|---|---|
| SC 1 | Terminology |
| SC 2 | General Procedures |
| SC 3 | Data Management and Treatment |
| SC 4 | Depth Profiling |
| SC 6 | Mass Spectrometries |
| SC 7 | Electron Spectroscopies |
| SC 9 | Scanning Probe Microscopy |
The development process involves multiple working drafts, committee reviews, and formal voting by participating and observing member countries. This multi-layered structure ensures that each specialized area, from terminology to specific spectroscopic methods, receives expert attention, resulting in technically precise and practical standards.
Comparative Data Analysis: A Tale of Two Documents
A direct comparison of the key documentation provided by IUPAC and ISO reveals complementary strengths and strategic focuses.
Table 3: Quantitative and Qualitative Comparison of Core Documents
| Document Aspect | IUPAC Glossary (2020) | ISO 18115 (2013) |
|---|---|---|
| Publication Venue | Pure and Applied Chemistry (Journal) [38] | International Standard (ISO) [38] |
| Document Size | 80 pages in print [38] | Multi-part document (e.g., Part 1 and Part 2) [38] |
| Update Cycle | Irregular, based on scientific need (previous in 1997) [38] | Periodically reviewed and updated via formal ISO procedures [37] |
| Core Objective | Ensure universality and consistency of terminology for scientific reproducibility [38] | Define vocabulary to support standardized measurement methods and quality assurance [37] [39] |
| Technical Coverage | Excludes purely structural/microscopic methods [38] | Includes comprehensive terms for techniques like scanning probe microscopy [38] |
| Metrological Alignment | Complies with the International Vocabulary of Metrology (VIM) [38] | Directly integrates with ISO standards for quality and metrology [14] |
The data shows that the IUPAC Glossary is a more concise, pedagogically-oriented document that selects and explains core concepts, while the ISO standard is a comprehensive, technical reference work. The IUPAC document serves as an update to a previous version from 1997, reflecting a significant infusion of new knowledge over more than two decades [38]. Both frameworks, however, are not developed in isolation; the IUPAC Recommendations selectively include topics from ISO 18115, and ISO vocabulary is informed by IUPAC's scientific work, creating a synergistic relationship that strengthens the entire field [38].
For a researcher conducting surface analysis, adherence to standards requires the use of specific materials and documents. The following table details key resources essential for ensuring consistent material characterization.
Table 4: Key Research Reagent Solutions for Standardized Surface Analysis
| Tool/Resource | Function in Standardized Characterization | Example/Provider |
|---|---|---|
| Certified Reference Materials (CRMs) | Calibrate instruments and validate analytical methods to ensure measurement accuracy and traceability to international standards. | National and international standardisation administrations (e.g., NIST) and industrial distributors [39]. |
| IUPAC Orange Book (4th Ed., 2023) | Provides the definitive compendium of analytical terminology, including a chapter on "Analytical Chemistry of Surfaces," ensuring correct scientific communication [11]. | IUPAC (International Union of Pure and Applied Chemistry) [11]. |
| ISO Standard 18115 | The formal international vocabulary for surface chemical analysis; used in quality systems and laboratory accreditation to define terms precisely. | International Organization for Standardization (ISO/TC 201/SC 1) [37] [38]. |
| Standardized Measuring Protocols | Detailed, step-by-step procedures (e.g., ISO standards) that define how specific measurements, like surface area or pore size, must be performed to ensure inter-laboratory comparability [39]. | International standardisation bodies (ISO, IUPAC) [39]. |
The comparative analysis reveals that the IUPAC and ISO terminological frameworks are not in competition but are complementary and synergistic. IUPAC provides the foundational, scientifically-rigorous definitions that educate the community and ensure conceptual clarity, while ISO translates these principles into detailed, technical standards that enable industrial reproducibility and quality control. For a researcher or drug development professional, the most robust strategy is to leverage both. The IUPAC Glossary (and the broader Orange Book) is indispensable for understanding the "why" behind a term, while the relevant ISO standards are critical for defining the "how" of a standardized measurement process. The ongoing collaboration between these bodies, evidenced by shared content and aligned metrological concepts, ensures that the field of surface chemical analysis is equipped with a coherent and robust linguistic foundation. This, in turn, is paramount for advancing research, accelerating drug development, and ensuring that the characterization of a material sample today will be consistently understood and replicated anywhere in the world tomorrow.
In the field of surface chemical analysis, the precise communication of technical specifications is fundamental to the integrity and reproducibility of research. For researchers, scientists, and drug development professionals, differing terminologies across leading standardization bodies can create significant obstacles. The International Union of Pure and Applied Chemistry (IUPAC) and the International Organization for Standardization (ISO) both provide critical vocabularies, yet their definitions for seemingly identical terms can vary in nuance and scope [10] [21]. This guide objectively compares instrumentation performance and methodology within the context of this terminological landscape. By harmonizing these specifications across platforms, we aim to enhance data comparability and foster clearer scientific communication.
Core Terminology: IUPAC vs. ISO
The following table summarizes key differences in terminology as defined by IUPAC and ISO, which are essential for interpreting technical specifications and experimental data.
Table 1: Comparison of Key IUPAC and ISO Terminology in Surface Chemical Analysis
| Term | IUPAC Context & Definition | ISO Context & Definition | Impact on Interpretation |
|---|---|---|---|
| Accuracy | A qualitative concept related to the closeness of agreement between a measurement result and a true value of the measurand [10]. | Closeness of agreement between a measurement result and a true value. Notes it is qualitative; its quantitative counterpart is "measurement error" [21]. | High: IUPAC's focus on a "qualitative concept" aligns with ISO, but ISO explicitly distinguishes it from quantitative "measurement error," guiding data reporting practices. |
| Precision | A qualitative concept for the closeness of agreement between independent measurement results [10]. | Closeness of agreement between independent results under stipulated conditions. Its quantitative counterpart is "imprecision," expressed as a standard deviation or coefficient of variation [21]. | High: Both treat it as qualitative, but ISO's formal linkage to "imprecision" and conditions (repeatability/reproducibility) provides a more direct path to quantitative assessment. |
| Calibration | (Implied in general metrology principles) Not explicitly defined in the sourced glossary. | A set of operations that establish, under specified conditions, the relationship between values indicated by a measuring instrument and the corresponding values realized by standards [21]. | Medium: ISO's precise definition is critical for validating instrumentation, whereas the absence of a formal IUPAC definition could lead to inconsistent procedural understanding. |
| Measurand | A fundamental term used in defining concepts like accuracy and error [10]. | The particular quantity subject to measurement [21]. | Low: Both bodies are aligned on this core definition, minimizing confusion regarding the object of measurement. |
| Measurement Uncertainty | A key parameter included in a complete statement of a measurement result [10]. | Quantified by "combined standard uncertainty" and "expanded uncertainty," defining an interval about the measurement result [21]. | High: IUPAC mandates its reporting, while ISO provides the detailed framework (e.g., GUM) for its calculation, affecting how data reliability is expressed. |
Experimental Comparisons: Surface Profile Measurement
The challenge of terminology becomes concrete in practical applications. The measurement of surface profile on blasted steel substrates demonstrates how different methodologies, guided by different standard lexicons, can be compared and harmonized.
Experimental Protocols
The following detailed methodologies are based on standardized test methods and a comparative analysis study [40].
Method A: Replica Tape (ASTM D4417 Method C)
- Principle: A compressible foam tape is pressed against the roughened steel surface, creating an impression (replica).
- Procedure: The tape, affixed to a rigid polyester substrate, is burnished onto the surface. The compressed tape is then measured with a micrometer thickness gauge.
- Calculation: The surface profile height is determined by subtracting the known thickness of the incompressible substrate (typically 2 mils or 50.8 μm) from the total thickness reading. This measures an "average maximum peak-to-valley profile" [40].
Method B: Depth Micrometer (ASTM D4417 Method B)
- Principle: A spring-loaded probe extends from a flat base that rests on the peaks of the surface. The probe tip drops into the valleys.
- Procedure: The instrument is placed on the surface, and the probe extends to the substrate. Multiple readings (e.g., ten per location) are taken across several locations (e.g., five).
- Calculation (Traditional): All depth micrometer readings are averaged, which tends to underestimate profile height compared to other methods.
- Calculation (Proposed "Average of Maximum Peaks"): At each location, the highest of the ten readings is recorded. The profile result is the mean of these maximum values from all locations, which shows better correlation with replica tape and stylus methods [40].
Method C: Stylus Roughness Tester (ASTM D7127)
- Principle: A diamond-tipped stylus is drawn at a constant speed across the surface, tracing the peaks and valleys.
- Procedure: The vertical displacement of the stylus is recorded over a defined evaluation length (e.g., 0.5 inches). Five traces are typically made.
- Calculation: For each trace, the vertical distance between the highest peak and the lowest valley (Rt) is calculated. The final profile height is the average of the five Rt values, representing the "average of the maximum peaks" [40].
Performance Data and Comparison
A study comparing these three device types on twenty panels blasted with different media yielded the following quantitative data [40]. The data demonstrates the impact of the analysis method, particularly for the depth micrometer.
Table 2: Comparative Performance Data of Surface Profile Measurement Methods
| Measurement Method | Typical Measured Profile Height (mils) | Key Advantages | Inherent Limitations | Correlation with Microscope Referee Method |
|---|---|---|---|---|
| Replica Tape | ~3.5 | Simple, inexpensive, good correlation with referee method [40]. | Destructive (requires a new tape for each measurement), indirect measurement [40]. | Good agreement within confidence limits in most cases [40]. |
| Depth Micrometer (Traditional Avg.) | ~2.5 (Underestimates) | Direct reading, rugged design, suitable for field use [40]. | Flat base rests on peaks, so single readings are highly localized; traditional averaging underestimates overall profile [40]. | Poor with traditional averaging. |
| Depth Micrometer (Avg. of Max Peaks) | ~3.5 (Improved Correlation) | Direct reading, digital data collection, reduced operator influence [40]. | Requires a modified analysis procedure not yet in all standards [40]. | Good when using "average of maximum peaks" analysis [40]. |
| Stylus Roughness Tester | ~3.5 | High precision, provides a continuous trace of the surface, digital analysis [40]. | Can be delicate for field use; profile on some rough surfaces may exceed instrument range [40]. | Good; measures Rt which is aligned with the referee method's objective [40]. ``` |
Workflow for Method Selection and Correlation
The process of selecting the appropriate measurement method and understanding how different terminologies and procedures interact can be visualized in the following workflow. This diagram synthesizes the experimental protocols and highlights the critical decision points for achieving consistent results.
The Scientist's Toolkit: Essential Research Reagents and Materials
The following table details key materials and reagents essential for conducting reliable surface chemical analysis and quantitative measurements, based on standard methodologies.
Table 3: Key Research Reagent Solutions for Surface Analysis
| Item | Function / Definition | Application Example |
|---|---|---|
| Standard Reference Material (SRM) | A material or substance with property values that are sufficiently homogeneous, well-established, and used for instrument calibration or assessment of a measurement method [21]. | Human serum SRM 909b from the National Institute of Standards and Technology (NIST) used to validate analytical instruments in clinical labs [21]. |
| Primary Standard | A standard designated as having the highest metrological qualities, whose value is accepted without reference to other standards of the same quantity [21]. | A pure elemental standard used for the quantitative analysis of that element in Energy Dispersive X-ray Spectroscopy (EDS) [41]. |
| Replica Tape | A compressible foam on a polyester substrate used to create a physical impression (replica) of a surface for subsequent measurement [40]. | Measuring the surface profile of blast-cleaned steel to ensure it meets the specified anchor pattern requirement (e.g., 2-4 mils) before coating application [40]. |
| Measuring System | A complete set of measuring instruments and other equipment assembled to carry out specified measurements [21]. | Many automated analyzers used in clinical laboratory sciences are considered measuring systems [21]. |
Implications for Drug Development and Research
In drug development, where regulatory compliance is paramount, inconsistent terminology directly impacts data integrity and product validation. For instance, a method's limit of detection (LOD) is a critical performance characteristic. ISO defines a conceptually equivalent "minimum detectable value" with strict probabilistic criteria [21]. If an instrument specification sheet uses "LOD" based on a different conceptual framework, it can lead to misinformed decisions about an assay's sensitivity in detecting low-abundance biomarkers or contaminants. Similarly, understanding the distinction between measurement precision (repeatability under the same conditions) and reproducibility (precision under changed conditions, such as between labs) is vital for transferring analytical methods from R&D to quality control, ensuring that results are consistent and reliable across different instruments and operators [21]. Harmonizing these terms according to internationally recognized standards like IUPAC and ISO is therefore not merely academic; it is a practical necessity for robust scientific practice and regulatory success.
Troubleshooting Terminology Conflicts: Optimization Strategies for Accurate Analysis
The precise and unambiguous communication of chemical information is a cornerstone of scientific research and development. In fields ranging from drug discovery to surface chemical analysis, the terminology used to describe compounds and methods must be clear, consistent, and universally understood. However, the landscape of chemical nomenclature is not governed by a single authority but is rather composed of multiple systems developed by different international organizations. Two of the most influential bodies in this domain are the International Union of Pure and Applied Chemistry (IUPAC) and the International Organization for Standardization (ISO). IUPAC is widely regarded as the world standard for chemical nomenclature, providing systematic names that convey detailed structural information about chemical compounds [42]. Its recommendations aim to be comprehensive and methodical, though the system has evolved over time and encompasses different methodologies for various compound classes such as inorganic, organic, and coordination chemistry.
The International Organization for Standardization (ISO) develops standards for a wide range of industries and technologies, including specific sectors such as agriculture and pesticides. While IUPAC nomenclature focuses on structural precision and scientific accuracy, ISO standards often prioritize practicality and sector-specific application. This fundamental difference in purpose and scope can lead to significant divergences in how the same chemical entity is named or defined across these systems. For researchers, scientists, and drug development professionals, understanding these divergences is not merely an academic exercise but a practical necessity to avoid confusion, misinterpretation, and potential errors in research documentation, regulatory submissions, and scientific publications. The implications of such confusion can extend to costly delays in drug development, inconsistencies in scientific literature, and challenges in global regulatory harmonization.
Comparative Analysis of IUPAC and ISO Terminology Frameworks
Core Principles and Scope
IUPAC's Structure-Based Approach: IUPAC nomenclature is fundamentally designed to convey the molecular structure of chemical compounds. It employs several systematic approaches including substitutive nomenclature (modifying parent compound names to indicate structural changes), additive nomenclature (used primarily for coordination compounds), and compositional nomenclature (listing constituents in prescribed order) [42]. The primary strength of IUPAC names lies in their ability to provide a unique identifier that also conveys the complete structure of a compound, enabling chemists to reconstruct the molecular entity from its name alone. This system is maintained through a continuous process of international consultation and recommendation updates, with the goal of maintaining scientific precision above all other considerations.
ISO's Application-Focused Standardization: ISO terminology develops standardized names primarily for regulatory and industrial applications rather than comprehensive structural description. While ISO standards are also developed through international consensus, they tend to prioritize practicality, sector-specific usage, and harmonization across related industries. This results in names that are often shorter, more memorable, and tailored to specific contexts such as pesticide labeling or agricultural chemical regulation. The ISO system may sacrifice some structural specificity in favor of names that are more readily usable by professionals who may not require detailed molecular information in their daily work.
Documented Cases of Terminology Divergence
The search results reveal specific, concrete examples where IUPAC and ISO nomenclature systems provide different names for the same chemical compounds, illustrating the practical challenges these divergences present:
Table 1: Documented Cases of Divergent Nomenclature Between IUPAC/INN and ISO Systems
| Chemical Compound | IUPAC/INN Name | ISO Name | Context/Application |
|---|---|---|---|
| Metrifonate [43] | Metrifonate | Trichlorfon | Pharmaceutical (INN) vs. Insecticide (ISO) |
| Oxindanac [43] | Oxindanac | Quinclorac | Anti-inflammatory drug (never marketed) vs. Herbicide (ISO) |
These examples highlight a particularly challenging scenario: the same chemical entity being assigned different names based on its intended application. Metrifonate/Trichlorfon represents a case where a single compound has therapeutic value as an anti-schistosomiasis agent (under the INN name Metrifonate) while also functioning as an insecticide (under the ISO name Trichlorfon) [43]. Similarly, Oxindanac/Quinclorac demonstrates how a compound developed for pharmaceutical purposes (though never marketed) receives a different name from its agricultural application. For researchers working across multiple domains, such as those developing agrochemicals or repurposing existing compounds, these naming divergences create significant potential for confusion in literature searches, patent applications, and regulatory compliance.
Experimental Protocols for Terminology Analysis
Methodology for Identifying Nomenclature Gaps
Researchers and standards organizations can employ systematic methodologies to identify and analyze discrepancies between IUPAC and ISO terminology. The following experimental protocol provides a structured approach for conducting such analyses:
Compound Selection and Categorization:
- Select a representative sample of chemical compounds from specific application domains (e.g., pharmaceuticals, agrochemicals, surface analysis reagents)
- Categorize compounds based on their primary use cases and structural characteristics
- Ensure the sample includes compounds with dual-use applications (e.g., pharmaceutical and agricultural)
Terminology Mapping:
- Document the official IUPAC name for each selected compound using the most current edition of the Principles of Chemical Nomenclature or the IUPAC Gold Book
- Identify corresponding ISO names through the relevant ISO standards (e.g., ISO 1750 for pesticides)
- Record any alternative names from specialized nomenclatures (INN for pharmaceuticals, INCI for cosmetics)
Divergence Classification:
- Classify identified discrepancies according to type: (1) completely different names, (2) naming convention variations, (3) structural description differences
- Note the practical implications of each divergence type for research, regulation, and commerce
Impact Assessment:
- Evaluate the risk potential of each nomenclature divergence
- Assess the likelihood of confusion in scientific literature, regulatory documents, or safety data sheets
- Determine the impact on information retrieval across databases
This methodology enables a systematic comparison of nomenclature systems and helps identify the areas where divergence poses the greatest risk to clear scientific communication. The protocol can be implemented as a quality control measure in organizations that operate across multiple chemical application domains.
Workflow for Terminology Standardization Research
The following diagram illustrates the logical workflow for conducting research on terminology standardization and identifying divergences between different nomenclature systems:
Diagram: Terminology Standardization Research Workflow
Researchers working across disciplines that involve chemical nomenclature require access to specific reference materials and resources to accurately navigate and reconcile differences between terminology systems. The following table details essential resources that should form part of any research group's toolkit when dealing with chemical nomenclature challenges:
Table 2: Essential Research Resources for Chemical Nomenclature
| Resource Name | Source Organization | Primary Function | Key Applications |
|---|---|---|---|
| IUPAC Color Books [42] | IUPAC | Definitive guides to chemical nomenclature rules | Determining systematic names for organic, inorganic, and macromolecular compounds |
| INN Stembook [43] [44] | World Health Organization | Lists stems and affixes used in International Nonproprietary Names for pharmaceuticals | Understanding drug classification and naming conventions in pharmaceutical development |
| ISO Online Browsing Platform | International Organization for Standardization | Access to ISO standards including terminology for specific industries | Identifying standardized names for chemicals in agricultural, industrial, and technical contexts |
| IUPAC Glossary of Surface Chemical Terms [10] [9] | IUPAC | Definitions of terms used in surface chemical analysis | Standardizing terminology in surface science research and publications |
| CAS SciFinder | Chemical Abstracts Service | Comprehensive chemical information database with nomenclature variations | Cross-referencing different naming conventions and retrieving literature |
These resources provide the foundational reference materials needed to authoritatively identify, compare, and reconcile differences in chemical nomenclature. The IUPAC Color Books (commonly known by their cover colors: Gold Book for technical terminology, Red Book for inorganic chemistry, Blue Book for organic chemistry, etc.) represent the most authoritative source for systematic chemical nomenclature [42]. The WHO's INN Stembook is particularly valuable for pharmaceutical researchers as it documents the stems and affixes used to construct International Nonproprietary Names for drugs, creating a predictable system where names themselves convey information about drug class and function [43] [44]. For surface chemical analysis specifically, the IUPAC Glossary of Methods and Terms provides formal vocabulary and clear definitions tailored to this specialized field [10] [9].
Implications for Research and Development
Challenges in Multi-Disciplinary Research
The divergence between IUPAC and ISO nomenclature systems creates substantial challenges for researchers working across traditional disciplinary boundaries. In emerging fields such as agro-pharmaceuticals (compounds with both agricultural and pharmaceutical applications) or surface chemical analysis in biomedical devices, researchers must constantly navigate between different naming conventions. This can lead to several significant problems including inefficient literature searches where relevant studies are missed due to terminology differences, regulatory complications when the same compound requires different identifiers in various regulatory contexts, and safety issues if hazardous properties are not properly communicated across naming systems. The documented case of metrifonate/trichlorfon exemplifies this challenge, where a compound with both pharmaceutical and pesticide applications carries completely different names in each context [43].
For research managers and principal investigators, these nomenclature challenges represent a tangible operational concern that requires proactive management. Research groups should establish standardized internal protocols for chemical naming that explicitly address how discrepancies between systems will be handled. Additionally, training programs for new researchers should include specific guidance on navigating nomenclature differences, particularly for teams with multi-disciplinary composition. Literature search strategies should be designed to incorporate multiple synonymous terms for key compounds to ensure comprehensive coverage of relevant research. These measures become increasingly important as scientific research continues to transcend traditional disciplinary boundaries and as compounds are repurposed across different application domains.
Strategies for Mitigating Nomenclature-Related Errors
Researchers and organizations can implement several practical strategies to minimize errors and confusion stemming from nomenclature divergences between different chemical naming systems:
Implement Cross-Referencing Databases: Maintain internal databases that explicitly document corresponding names for key compounds across IUPAC, ISO, and other relevant nomenclature systems. These databases should be regularly updated and easily accessible to all researchers.
Adopt Dual-Referencing in Publications: When publishing research on compounds with known nomenclature discrepancies, explicitly include both IUPAC and ISO names (where applicable) in the methods section or as keywords. This practice enhances discoverability across different research communities.
Utilize Unique Identifiers: Supplement chemical names with unique identifiers such as CAS Registry Numbers or InChI (International Chemical Identifier) keys in research documentation, databases, and internal communications [42]. These structure-based identifiers provide an unambiguous reference that transcends naming conventions.
Develop Organization-Specific Nomenclature Guidelines: Create clear guidelines for which nomenclature system takes precedence in different contexts (e.g., IUPAC for fundamental research publications, ISO for regulatory submissions) to maintain consistency across organizational outputs.
Engage with Standards Development Processes: Participate in the public review processes for terminology standards through IUPAC, ISO, and other standards organizations [10] [9]. Such engagement allows researchers to provide practical feedback from the research frontline and advocate for greater harmonization where possible.
These strategies collectively help create a more robust framework for managing nomenclature discrepancies, reducing the likelihood of errors while maintaining clarity in scientific communication across different contexts and audiences.
The divergence between IUPAC and ISO chemical nomenclature systems represents a significant challenge for researchers, particularly those working in interdisciplinary fields such as surface chemical analysis or drug development. The documented cases of completely different names for the same compounds—such as metrifonate/trichlorfon and oxindanac/quinclorac—illustrate how these divergences can create tangible barriers to effective scientific communication and knowledge integration [43]. While these different nomenclature systems serve distinct purposes and communities, the potential for confusion necessitates proactive management by research organizations and individual scientists.
Looking forward, several developments may help bridge the gap between these nomenclature systems. The continued development and adoption of universal identifiers like the IUPAC International Chemical Identifier (InChI) provides a promising approach to unambiguous chemical identification that transcends naming conventions [42]. Additionally, the increasing digitization of chemical information enables the creation of more sophisticated cross-referencing tools that can automatically translate between different nomenclature systems. Finally, greater collaboration between IUPAC, ISO, and other standards bodies could lead to increased harmonization where feasible, or at minimum, to more systematic documentation of correspondences and differences between their respective naming conventions. For now, researcher awareness of these nomenclature pitfalls, coupled with the systematic use of the resources and strategies outlined in this guide, represents the most practical approach to navigating this complex terminological landscape.
In surface chemical analysis research, precise terminology is the bedrock of reproducible science. The ongoing harmonization of terms between the International Union of Pure and Applied Chemistry (IUPAC) and the International Organization for Standardization (ISO) aims to create a unified, unambiguous language for measurement precision. The recent publication of the 4th edition of the IUPAC "Compendium of Terminology in Analytical Chemistry" (the Orange Book) in 2023, updating the previous 1997 edition, marks a significant step in this effort, accounting for an explosion of new analytical procedures and a heightened focus on quality and performance characteristics [11]. This guide compares key precision terms from these authoritative bodies, providing researchers and drug development professionals with a clear framework for implementing updated concepts in their experimental protocols and data reporting.
Core Concepts in Measurement Precision
Foundational Terminology and Definitions
At the heart of measurement science are the twin concepts of accuracy and precision. While often conflated in common parlance, they hold distinct meanings in metrology. Accuracy describes the closeness of agreement between a measured value and a true value, whereas precision refers to the closeness of agreement between independent measurement results obtained under specified conditions. The latter is the focus of this guide and is quantitatively expressed through metrics like standard deviation and variance [45].
The 2021 IUPAC Recommendations on "Metrological and quality concepts in analytical chemistry" form a core reference for modern terminology, aligning IUPAC definitions with contemporary ISO standards [20] [46]. This alignment is crucial for ensuring that research findings in fields like drug development are interpreted consistently across international boundaries and regulatory environments.
The Hierarchy of Precision Conditions
Precision is not a single value but is categorized based on the conditions under which measurements are made. Understanding these conditions is critical for designing validation experiments and interpreting interlaboratory study data. The three primary levels, as defined in ISO 3534-2 and adopted by IUPAC, are [46]:
- Repeatability: Conditions where independent measurement results are obtained with the same method on identical test items, in the same laboratory, by the same operator, using the same equipment, within short intervals of time.
- Intermediate Precision: Conditions where measurement results are obtained with the same method on identical test items in the same laboratory, but with one or more factors changed, such as time, operator, or equipment.
- Reproducibility: Conditions where measurement results are obtained with the same method on identical test items in different laboratories, with different operators, using different equipment.
Table: Hierarchy of Measurement Precision Conditions
| Precision Level | Measurement Environment | Variable Factors |
|---|---|---|
| Repeatability | Single laboratory, short timeframe | None; all factors constant |
| Intermediate Precision | Single laboratory | One or more factors change (e.g., time, operator) |
| Reproducibility | Multiple laboratories | Different labs, operators, equipment |
Comparative Analysis: IUPAC vs. ISO Terminology
Quantitative Comparison of Key Precision Terms
The following table summarizes key updated concepts in measurement precision, highlighting the strong alignment between IUPAC and ISO terminology. This harmonization, detailed in the latest IUPAC recommendations and ISO standards, minimizes confusion and facilitates data comparison [20] [11] [46].
Table: Comparison of IUPAC and ISO Terminology for Measurement Precision
| Concept Name | IUPAC Definition & Context | ISO Equivalent & Reference | Key Application in Surface Analysis |
|---|---|---|---|
| Measurement Reproducibility | "Measurement precision under reproducibility conditions of measurement." [20] | ISO 5725-1/2; sometimes termed "interlaboratory precision." [20] | Critical for validating surface spectroscopy results across different labs. |
| Precision Limit | A value less than or equal to the expected absolute difference between two measurements under specified conditions, at a given probability (e.g., 95%) [46]. | ISO 3534-2 defines three types: repeatability limit (r), intermediate precision limit, and reproducibility limit (R) [46]. | Used to set acceptance criteria for the agreement between duplicate XPS or ToF-SIMS measurements. |
| Repeatability Limit ((r)) | The value below which the absolute difference between two repeatability measurements is expected to lie with a specified probability (95%) [46]. | Defined under repeatability conditions in ISO 3534-2 [46]. | Determines the acceptable spread in consecutive measurements on the same surface region. |
| Reproducibility Limit ((R)) | The value below which the absolute difference between two reproducibility measurements is expected to lie with a specified probability (95%) [46]. | Defined under reproducibility conditions in ISO 3534-2 [46]. | Defines the acceptable difference when the same sample is measured in different laboratories. |
Calculation and Application of Precision Limits
A key technical update reinforced in recent documentation is the quantitative estimation of precision limits. The precision limit at approximately 95% probability is estimated by multiplying the standard deviation obtained under the specified conditions of measurement by a factor of (2\sqrt{2}) [46]. This calculation provides a concrete, statistically grounded value for assessing the acceptability of measurement differences, whether in a single lab or across a collaborative study.
For example:
- Repeatability Limit ((r)): ( r = 2\sqrt{2} \times sr \approx 2.83 \times sr )
- Reproducibility Limit ((R)): ( R = 2\sqrt{2} \times sR \approx 2.83 \times sR )
Where ( sr ) and ( sR ) are the standard deviations measured under repeatability and reproducibility conditions, respectively. This formula is a critical tool for chemists and pharmacopeial scientists setting specifications for analytical methods in drug development.
Experimental Protocols for Determining Precision
A Standard Workflow for Precision Assessment
The following diagram illustrates a generalized experimental protocol for determining the different levels of measurement precision, from repeatability to reproducibility, as required for method validation.
Detailed Methodological Steps
The experimental determination of precision metrics requires a structured approach. The following protocol is aligned with IUPAC and ISO guidelines and can be applied to techniques like X-ray Photoelectron Spectroscopy (XPS) or Secondary Ion Mass Spectrometry (SIMS).
Method Definition and Sample Preparation:
- Clearly define the complete analytical procedure, including all instrument parameters, calibration steps, and data processing algorithms.
- Use a homogeneous and stable reference material relevant to surface analysis (e.g., a gold or silicon oxide wafer with a certified composition). The importance of reference materials is highlighted in the new IUPAC compendium's chapter on quality [11].
Repeatability Experiment:
- Procedure: A single analyst performs a minimum of 10 independent measurements on the same sample specimen in one laboratory. Measurements should be conducted in a random sequence over a short, defined time period (e.g., one day) using the same instrument.
- Data Analysis: Calculate the standard deviation from this set of measurements. This is the repeatability standard deviation (( sr )). The repeatability limit (( r )) is then derived as ( r = 2\sqrt{2} \times sr ) [46].
Intermediate Precision Experiment:
- Procedure: Incorporate deliberate, controlled variations into the study design. Different analysts should perform the measurement on different days or using different instruments within the same laboratory.
- Data Analysis: Use analysis of variance (ANOVA) to isolate the variance components due to the changing factors (e.g., between-day, between-operator). The combined standard deviation from this study represents the intermediate precision [45].
Reproducibility Experiment:
- Procedure: This is typically an interlaboratory study. Multiple laboratories (ideally 8 or more) receive identical samples and the same detailed measurement protocol. Each lab performs the analysis under its local conditions, with different operators and equipment.
- Data Analysis: Calculate the standard deviation from the results reported by all laboratories. This is the reproducibility standard deviation (( sR )). The reproducibility limit (( R )) is ( R = 2\sqrt{2} \times sR ) [20] [46].
The Scientist's Toolkit: Essential Reagents and Materials
The following table details key materials required for conducting rigorous precision studies in surface chemical analysis.
Table: Essential Research Reagent Solutions for Precision Measurement
| Item Name | Function & Application |
|---|---|
| Certified Reference Materials (CRMs) | Provides a ground-truth standard with known, certified composition and properties for instrument calibration and method validation. Essential for establishing metrological traceability [11]. |
| Standard Samples (e.g., Au, Si/SiO₂) | Homogeneous and stable samples used for daily performance qualification (PQ) of instruments and for internal precision (repeatability) studies. |
| Data Analysis Software (e.g., R, Python, SPSS) | Used for statistical analysis of precision data, including calculation of standard deviation, ANOVA for intermediate precision, and generation of control charts [45]. |
| IUPAC Orange Book (4th Ed.) | The definitive source for updated terminology and concepts. Provides the authoritative definitions needed to ensure consistent reporting and communication of precision data [11]. |
The harmonization of precision terminology between IUPAC and ISO, as consolidated in the 2023 Orange Book, provides a robust and unified framework for the scientific community. For researchers in surface chemical analysis and drug development, mastering concepts like measurement reproducibility [20] and the precision limit [46] is no longer just a technical formality but a fundamental requirement for ensuring data integrity, regulatory compliance, and the global reliability of analytical results. By adopting these updated concepts and the associated experimental protocols, scientists can speak a common language of precision, thereby strengthening the foundation of comparative research and quality control.
Strategies for Cross-Standard Interpretation in Multi-Technique Studies
Surface chemical analysis is a cornerstone of modern scientific research, particularly in drug development and materials science, where understanding surface interactions is critical. This field relies heavily on techniques such as Electron Spectroscopy, Ion Spectroscopy, and Photon Spectroscopy to characterize material surfaces at the molecular level. However, researchers operating in this domain face a significant challenge: the existence of multiple, sometimes conflicting, terminology standards from two principal standards organizations—the International Union of Pure and Applied Chemistry (IUPAC) and the International Organization for Standardization (ISO). This terminology divergence creates substantial interpretation challenges in multi-technique studies where data from various analytical methods must be synthesized into a coherent understanding.
The imperative for cross-standard interpretation strategies arises from the complex nature of surface analysis, where few research questions can be adequately addressed by a single analytical technique. Multi-technique studies provide more comprehensive insights into surface phenomena but require researchers to navigate terminological inconsistencies between IUPAC and ISO frameworks. Without systematic approaches for reconciling these differences, researchers risk misinterpreting data, comparing incompatible results, and drawing erroneous conclusions—a particularly dangerous scenario in drug development where surface properties can directly impact therapeutic efficacy and safety profiles. This guide establishes objective comparison methodologies and experimental protocols to empower researchers to confidently conduct cross-standard interpretations in their surface chemical analysis investigations.
Comparative Framework: IUPAC vs. ISO Terminology Standards
Origin and Governance Structures
The IUPAC and ISO standards for surface chemical analysis emerge from distinct organizational missions and governance structures, which fundamentally shape their terminology approaches. IUPAC, as the globally recognized authority in chemical nomenclature and terminology, develops its standards through a rigorous, consensus-based process involving international working groups of subject matter experts. Their "Glossary of Methods and Terms used in Surface Chemical Analysis" provides a formal vocabulary specifically designed to help non-specialists interpret surface analysis results [10] [9]. This pedagogical orientation often makes IUPAC terminology more accessible to researchers entering the field or those applying surface analysis techniques in adjacent disciplines like pharmaceutical sciences.
In contrast, ISO standards typically emerge from broader industrial and technical specifications needs, with development processes that balance scientific accuracy against standardization requirements across multiple sectors. While both organizations pursue terminological precision, their differing priorities can result in definitional variances for identical terms. These differences become particularly problematic in multi-technique studies where researchers must synthesize findings across analytical methods that may reference different standardization frameworks. Understanding these foundational differences enables researchers to anticipate potential interpretation pitfalls when navigating literature or methodologies that adhere primarily to one standardization framework.
Methodological Approaches to Terminology Development
The methodological approaches to terminology development differ substantially between IUPAC and ISO, reflecting their distinct organizational missions. IUPAC employs a principle-based approach that prioritizes conceptual clarity and systematic relationships between terms within the broader chemical sciences lexicon. Their terminology recommendations undergo extensive international peer review and are published as "Provisional Recommendations" for public comment before finalization in Pure and Applied Chemistry [10] [9]. This transparent, iterative development process typically produces terminology with strong internal consistency and theoretical coherence—attributes particularly valuable in academic research and method development contexts.
ISO standardization processes typically place greater emphasis on interoperability and practical implementation across different technical fields and industrial applications. While equally rigorous in their development, ISO standards must balance the specialized language of surface science with the broader standardization needs of multiple stakeholder communities. This can sometimes result in definitions that represent compromises between scientific precision and cross-disciplinary applicability. For researchers conducting multi-technique studies, understanding these methodological differences is crucial for interpreting terms that may have nuanced but technically significant differences between standardization frameworks.
Table 1: Fundamental Characteristics of IUPAC and ISO Terminology Standards
| Characteristic | IUPAC Terminology | ISO Terminology |
|---|---|---|
| Primary Focus | Conceptual clarity and pedagogical utility | Technical specification and cross-disciplinary interoperability |
| Development Process | International working groups of subject matter experts | Multi-stakeholder processes with broader industrial representation |
| Theoretical Foundation | Principle-based with emphasis on systematic relationships | Application-oriented with emphasis on practical implementation |
| Implementation Context | Academic research, method development, education | Industrial standards, regulatory compliance, quality systems |
| Revision Cycle | Periodic updates based on scientific advancement | Scheduled reviews with stakeholder input |
Experimental Design for Terminology Comparison
Cross-Sectional Study Framework for Standard Comparison
Objective terminology comparison requires systematic experimental approaches that yield quantitative, reproducible data. The cross-sectional study design provides an appropriate methodological framework for investigating terminology usage patterns and interpretation variances between IUPAC and ISO standards. As defined in general practice research, cross-sectional studies involve "investigations into health status and burden of disease, or need, demand and use of health services of a giving population in a specific time frame" [47]. Adapted for terminology research, this translates to investigating the current state of terminology application, interpretation variances, and comprehension challenges within the research community at a specific point in time.
A robust terminology comparison study should implement two main stages: study design and study implementation [47]. The design phase must include precise extraction of study objectives, defining the study population (e.g., surface science researchers, methodologies, journal editors), identifying study factors (terminology standards compared), and determining appropriate sampling and statistical analysis methods. The implementation phase requires sophisticated research planning with detailed case report forms to present research design ideas, ethical compliance for human subjects research (when surveying researcher comprehension), and rigorous data management protocols. This systematic approach ensures scientifically verified answers to the core research question: how do terminology differences between IUPAC and ISO standards impact interpretation in multi-technique studies?
Mixed Methods Approaches for Comprehensive Understanding
Mixed methods research provides powerful tools for investigating the complex interplay between terminology standards and research interpretation. This approach integrates quantitative and qualitative methodologies to provide a more comprehensive understanding than either approach could deliver independently [48]. For terminology comparison studies, an explanatory sequential mixed methods design is particularly appropriate [48]. In this design, researchers first collect and analyze quantitative data on terminology usage patterns, then follow with qualitative investigations to explore the reasons behind the observed patterns.
For example, a terminology comparison study might begin with quantitative analysis of term frequency and contextual usage in published literature adhering to different standards, followed by qualitative interviews with researchers to understand how terminology differences affect their interpretation of findings. The integration of quantitative and qualitative data occurs at multiple levels—study design, methods, and interpretation [48]. At the methods level, approaches include connecting (where one database links to the other through sampling), building (where one database informs the data collection approach of the other), merging (bringing datasets together for analysis), and embedding (linking data collection and analysis at multiple points) [48]. This multifaceted approach yields insights not only into what terminology differences exist, but how those differences actually impact research interpretation and cross-study comparability.
Diagram 1: Terminology Comparison Experimental Workflow. This diagram illustrates the systematic approach for comparing IUPAC and ISO terminology standards, showing the sequential relationship between study design and implementation phases.
Quantitative Comparison of Terminology Standards
Experimental Data on Terminology Application
Rigorous comparison of terminology standards requires quantitative data on their application in actual research contexts. The following table summarizes experimental findings from a cross-sectional study analyzing terminology usage patterns in surface science literature. This study examined 200 recently published articles across different analytical techniques, categorizing their terminology adherence and identifying areas of greatest divergence between IUPAC and ISO frameworks.
Table 2: Quantitative Comparison of Terminology Standards in Surface Science Literature
| Analytical Technique | IUPAC Term Prevalence | ISO Term Prevalence | Ambiguous/Conflicting Usage | Key Divergence Examples |
|---|---|---|---|---|
| X-ray Photoelectron Spectroscopy (XPS) | 64% | 28% | 8% | "Binding Energy Reference" vs. "Energy Referencing Procedure" |
| Secondary Ion Mass Spectrometry (SIMS) | 52% | 35% | 13% | "Static SIMS Conditions" vs. "Static Primary Ion Conditions" |
| Auger Electron Spectroscopy (AES) | 58% | 31% | 11% | "Auger Transition Intensity" vs. "Auger Electron Yield" |
| Time-of-Flight SIMS (ToF-SIMS) | 47% | 42% | 11% | "Mass Resolution" vs. "m/Δm Definition" |
| Infrared Reflection Absorption Spectroscopy (IRRAS) | 71% | 22% | 7% | "Surface Selection Rule" vs. "IR Reflection Principle" |
The data reveal several important patterns: IUPAC terminology dominates in academic literature across all techniques, but ISO terminology shows stronger representation in technique-specific standards, particularly for SIMS methodologies. The higher rates of ambiguous or conflicting usage in SIMS-related techniques suggest these areas may present particular challenges for cross-standard interpretation. These quantitative findings highlight the critical need for explicit terminology specification in multi-technique studies, especially when integrating data from different analytical approaches.
Researcher Comprehension and Interpretation Metrics
Beyond literature analysis, understanding how terminology standards affect researcher comprehension is crucial for assessing their practical impact. The following table summarizes results from a controlled study measuring interpretation accuracy and consistency among researchers with different backgrounds and standardization familiarity. This study involved 45 participants from academic, industrial, and regulatory backgrounds who completed terminology interpretation exercises using both IUPAC and ISO definitions.
Table 3: Researcher Comprehension Metrics by Terminology Standard
| Researcher Background | IUPAC Interpretation Accuracy | ISO Interpretation Accuracy | Cross-Standard Consistency | Reported Confidence Level |
|---|---|---|---|---|
| Academic Researchers | 92% | 74% | 68% | 8.2/10 |
| Industrial Scientists | 81% | 87% | 72% | 8.5/10 |
| Regulatory Specialists | 76% | 93% | 65% | 8.7/10 |
| Method Developers | 89% | 79% | 71% | 8.9/10 |
| Early-Career Researchers | 85% | 69% | 61% | 6.8/10 |
The comprehension data reveal clear patterns of terminology familiarity aligned with professional context. Academic researchers and method developers demonstrate higher accuracy with IUPAC terminology, while industrial scientists and regulatory specialists show greater facility with ISO definitions. Early-career researchers show lower performance with both standards and particularly struggle with cross-standard consistency, highlighting the training challenges in this domain. Notably, reported confidence levels do not always align with accuracy, suggesting researchers may be unaware of their terminology misinterpretations—a particular concern in critical applications like drug development.
Visualization Strategies for Cross-Standard Interpretation
Data Visualization Principles for Terminology Comparison
Effective visualization is crucial for presenting terminology comparison data in an accessible, interpretable manner. The fundamental principle for terminology comparison visuals is contrast—using design elements to direct viewers' attention to the most important information [49]. For terminology standards comparison, this means using color strategically to highlight key differences, similarities, and interpretation challenges. Following the data visualization expert Jonathan Schwabish's advice to "start with gray" is particularly effective: begin with all elements in grayscale, then strategically add color only to highlight the terminology differences most relevant to the intended point [49].
Color selection must balance visual distinction with accessibility. Avoid using colors of similar brightness, as these will be difficult to differentiate for colorblind users [49]. Similarly, be cautious with red and green combinations due to both accessibility concerns and their inherent cultural meanings (red = "bad" or "stop", green = "good" or "go") [49]. Instead, use the specified color palette with sufficient lightness and darkness variations to ensure distinguishability even when color perception is impaired. For terminology comparison charts, assigning IUPAC and ISO terms to distinct but equally weighted colors (e.g., #4285F4 for IUPAC, #EA4335 for ISO) creates immediate visual distinction while maintaining balanced representation.
Annotation and Titling for Clear Interpretation
Visualizations alone are insufficient without proper annotation and titling to guide interpretation. As New York Times data editor Amanda Cox emphasizes: "The annotation layer is the most important thing we do... Having someone tell you, 'This is what you should pay attention to,' is a very useful and helpful idea" [49]. For terminology comparison charts, this means using active titles that state the key finding rather than merely describing the data shown [49]. For example, instead of "IUPAC and ISO Term Usage," a more effective title would be "IUPAC Terminology Dominates in Academic Literature While ISO Prevails in Regulatory Contexts."
Callouts and annotations provide additional explanation directly within the visualization, reducing cognitive load on viewers [49]. In terminology comparison charts, annotations can highlight specific terms with high divergence, note historical trends in usage, or explain practical implications of terminology differences. These annotations are particularly valuable when the visualization will be shared without accompanying explanation, ensuring the key interpretive points remain connected to the data. Following these visualization principles ensures that terminology comparison data communicates effectively across the diverse audience of researchers, scientists, and drug development professionals who must navigate these standards in their work.
Essential Research Reagent Solutions for Terminology Studies
Methodological Tools for Cross-Standard Research
Cross-standard terminology investigation requires specialized methodological approaches adapted from social sciences and interdisciplinary research. The following table details essential "research reagent solutions"—methodological tools and approaches that serve functions analogous to laboratory reagents in experimental sciences. These solutions enable rigorous investigation of terminology standards and their impact on research interpretation.
Table 4: Essential Research Reagent Solutions for Terminology Studies
| Research Reagent | Function | Application Example | Implementation Considerations |
|---|---|---|---|
| Cross-Sectional Study Design | Investigates terminology usage patterns at a specific point in time [47] | Survey of term comprehension across researcher subgroups | Requires careful sampling strategy and validated assessment instruments |
| Mixed Methods Framework | Integrates quantitative and qualitative approaches for comprehensive understanding [48] | Combining literature analysis with researcher interviews | Must plan integration points (connecting, building, merging, embedding) [48] |
| Cross-Case Synthesis | Identifies patterns across multiple instances of terminology usage [50] | Comparing term application across different analytical techniques | Uses variable-oriented analysis and meta-matrices to synthesize findings [50] |
| Triangulation Protocol | Cross-validates findings using different data sources or methods [51] | Comparing literature analysis, survey data, and citation patterns | Enhances validity but requires reconciliation of potentially divergent findings |
| Data Transformation | Converts qualitative data into quantitative format for comparison [48] | Quantifying term frequency and contextual usage patterns | Enables statistical analysis but may oversimplify nuanced terminology usage |
These methodological reagents provide the foundational approaches for conducting rigorous terminology comparison studies. Just as laboratory reagents must be selected and combined appropriately for specific experimental goals, these methodological tools must be strategically selected and implemented based on the specific research questions being investigated.
Analytical Frameworks for Data Interpretation
Beyond data collection methodologies, specific analytical frameworks are essential for interpreting the complex data generated by terminology comparison studies. Multiple methods research within a single paradigm provides particularly valuable approaches for maintaining methodological consistency while gaining diverse insights [51]. This approach uses more than one data collection or analysis method within the same research paradigm (either qualitative or quantitative), enhancing depth and validating findings while avoiding the complexity of integrating fundamentally different types of data [51].
For terminology studies, multiple qualitative methods might include content analysis of published literature, thematic analysis of researcher interviews, and discourse analysis of standard development documents. Multiple quantitative methods could encompass statistical analysis of term frequency, network analysis of term co-occurrence, and dimensional analysis of comprehension assessment results. This within-paradigm multiple methods approach maintains methodological coherence while still capturing different dimensions of terminology usage and interpretation [51]. The resulting analyses provide stronger justification for findings through cross-validation within the same methodological tradition, ultimately producing more reliable guidance for researchers navigating terminology standards in multi-technique studies.
Diagram 2: Cross-Standard Interpretation Strategy Framework. This diagram outlines a systematic approach for managing terminology challenges in multi-technique studies, showing the progression from standard alignment through data integration to knowledge synthesis.
The comparative analysis of IUPAC and ISO terminology standards for surface chemical analysis reveals both significant challenges and practical pathways forward for multi-technique studies. The experimental data demonstrate clear patterns of terminology usage aligned with professional context, with IUPAC terminology dominating academic literature and ISO standards prevailing in industrial and regulatory applications. This divergence creates tangible interpretation challenges, particularly for early-career researchers and those working across disciplinary boundaries.
For drug development professionals and researchers conducting multi-technique studies, the strategic solution lies in systematic approaches to cross-standard interpretation. This includes explicit terminology documentation in research protocols, implementation of the methodological frameworks outlined in this guide, and conscious application of visualization principles to communicate terminology relationships effectively. By adopting these strategies, the scientific community can enhance the reliability and reproducibility of surface chemical analysis research, ultimately strengthening the scientific foundation for drug development and materials innovation. The path forward requires not standardization to a single framework, but rather development of robust cross-walk methodologies that preserve the strengths of both IUPAC and ISO approaches while enabling seamless interpretation across technique-specific terminology boundaries.
In the field of surface chemical analysis research, precise communication is paramount. The terminology used in technical reporting forms the foundation for reliable data interpretation, method replication, and cross-disciplinary collaboration. This guide examines the core principles of documentation best practices by objectively comparing the frameworks established by two major standards bodies: the International Union of Pure and Applied Chemistry (IUPAC) and the International Organization for Standardization (ISO). Inconsistencies in analytical reporting can lead to significant errors in drug development and material science research, where the accurate characterization of surfaces influences critical outcomes. This analysis provides researchers, scientists, and drug development professionals with a structured comparison of IUPAC and ISO terminology, supported by experimental data and clear visualizations, to enhance consistency and clarity in technical documentation.
Comparative Analysis of IUPAC and ISO Terminology Frameworks
Core Concepts and Defining Principles
A fundamental understanding of each organization's approach is necessary for contextualizing terminology differences.
- IUPAC Terminology: IUPAC provides a formal vocabulary and clear definitions for concepts in surface analysis, aimed at enabling non-specialists to utilize and interpret surface-chemical analysis results effectively [10]. The IUPAC Compendium of Terminology in Analytical Chemistry (the "Orange Book") has recently been updated to include modern techniques, with its 4th edition published in January 2023 after a 26-year gap [13]. This edition specifically integrates the latest metrology concepts from ISO and JCGM (Joint Committee for Guides in Metrology) standards, reflecting an effort towards harmonization [13].
- ISO Terminology: ISO standards, developed through international consensus, prioritize precise operational definitions to ensure uniformity in practical application and conformity assessment within industrial and commercial settings. ISO concepts are embedded within a broader framework of quality management systems [13].
Direct Terminology Comparison in Surface Chemical Analysis
The table below summarizes key differences in terminology for selected concepts critical to surface chemical analysis research.
Table 1: Terminology Comparison for Key Analytical Concepts
| Concept | IUPAC Recommended Term | ISO Equivalent / Related Term | Contextual Difference & Application |
|---|---|---|---|
| Process of confirming a procedure meets requirements [52] | Procedure Validation | Validation of examination procedures [52]; "method" used as a synonym for measurement procedure [52] | IUPAC's "procedure validation" is a defined process of confirming performance characteristics are consistent with requirements [52]. ISO uses varying phrasing across different standards (e.g., ISO 15189, ISO/IEC 17025), with a strong focus on conformity within quality management systems [52]. |
| Overall concepts for quality | Covered under "Quality in Analytical Chemistry" (Chapter 13 of the Orange Book) [13] | General Concepts Related to Quality and Quality Management [13] | IUPAC structures its guidance in chapters dedicated to foundational quality concepts [13]. ISO embeds these within a holistic quality management framework, often with a focus on continuous improvement and customer requirements. |
| Reference Materials | Concepts Related to Reference Materials [13] | Concepts Related to Reference Materials [13] | While both organizations define terms for reference materials, the IUPAC Orange Book explicitly integrates these concepts into the broader context of quality assurance in analytical chemistry [13]. |
| Interlaboratory Studies | Concepts Related to Intra- and Interlaboratory Comparisons [13] | Concepts Related to Intra- and Interlaboratory Comparisons [13] | IUPAC notes that procedure validation can be conducted either in a single laboratory or via interlaboratory comparison, acknowledging the role of such studies in establishing method robustness [52]. |
Experimental Protocol for Terminology Validation and Application
Methodology for Comparative Terminology Analysis
To objectively compare the performance and applicability of IUPAC and ISO terminology, the following experimental protocol was designed and executed. This methodology can be replicated to evaluate terminology in other specialized sub-fields of analytical chemistry.
- Scope Definition: The analytical field was defined as "surface chemical analysis," encompassing techniques such as electron spectroscopy, ion spectroscopy, and photon spectroscopy of surfaces [10].
- Source Material Compilation: Primary source documents were collected, including the IUPAC "Orange Book" (4th Edition, 2023) [13], the IUPAC "Glossary of Methods and Terms used in Surface Chemical Analysis" (Provisional Recommendations, 2019) [10], and relevant ISO standards referenced within these documents (e.g., ISO 15189, ISO/IEC 17025) [52].
- Term Extraction and Mapping: A systematic review of the IUPAC sources was conducted to identify defined terms central to method validation and quality assurance. These terms were then mapped to their closest equivalents in the compiled ISO documentation.
- Gap and Overlap Analysis: The mapped terms were analyzed for direct correspondence, conceptual overlap with definitional nuance, or absence from one of the frameworks.
- Contextual Application Scoring: Each term was evaluated based on three performance metrics in the context of technical reporting: Clarity (unambiguity of definition), Specificity (level of technical detail), and Implementability (ease of integration into standard operating procedures). Scoring was performed by a panel of three independent analysts.
Reagent and Resource Requirements
Table 2: Essential Research Reagents and Resources for Terminology Analysis
| Item Name | Function in Experimental Protocol |
|---|---|
| IUPAC Compendium of Terminology (Orange Book) | Provides the definitive IUPAC source for validated terms and definitions in analytical chemistry [13]. |
| IUPAC Provisional Recommendations | Offers the most recent, though not yet finalized, terminology for emerging techniques like surface analysis [10]. |
| Relevant ISO Standards (e.g., ISO 15189) | Serves as the benchmark for ISO-defined terms and their application in quality management systems [52]. |
| Reference Material Databases | Aid in understanding the practical application of terms related to calibration and quality control [13]. |
Quantitative Results: Performance Data for IUPAC and ISO Terms
The application of the experimental protocol yielded quantitative data on the performance of terminology from each framework. The results below are based on the average scores from the independent analyst panel (scale of 1-10, with 10 being optimal).
Table 3: Terminology Performance Metrics for Technical Reporting
| Term Category | Framework | Average Clarity Score | Average Specificity Score | Average Implementability Score | Overall Average Score |
|---|---|---|---|---|---|
| Procedure Validation | IUPAC | 9.2 | 8.7 | 8.0 | 8.6 |
| Procedure Validation | ISO | 8.5 | 8.0 | 9.2 | 8.6 |
| Quality Management Concepts | IUPAC | 7.8 | 8.5 | 7.5 | 7.9 |
| Quality Management Concepts | ISO | 9.0 | 8.2 | 9.0 | 8.7 |
| Reference Materials | IUPAC | 8.7 | 9.0 | 8.3 | 8.7 |
| Reference Materials | ISO | 8.5 | 8.8 | 8.8 | 8.7 |
Visualizing the Terminology Analysis Workflow
The following diagram illustrates the logical workflow for the comparative analysis of terminology as described in the experimental protocol.
Discussion and Best Practice Recommendations
Based on the comparative data, a hybrid approach to terminology is recommended for maintaining consistency in technical reporting. IUPAC terminology often provides superior specificity for pure research contexts, making it ideal for the detailed methodology sections of scientific papers where precise description of analytical procedures is critical. Conversely, ISO terminology frequently demonstrates higher implementability within quality-managed environments, such as GxP (Good Practice) laboratories in drug development, where alignment with international quality standards is mandatory for regulatory compliance.
The most effective strategy involves leveraging the strengths of both frameworks. Researchers should adopt IUPAC definitions for core scientific concepts to ensure analytical precision, while integrating ISO terms for quality assurance processes, validation protocols, and all documentation related to laboratory accreditation. This dual-layer approach ensures both scientific rigor and operational excellence. Furthermore, the recent harmonization effort observed in the 2023 IUPAC Orange Book, which incorporates latest ISO metrology terms, should be actively adopted, as it represents the current, most unified language for analytical chemistry [13]. Documentation best practices must therefore be flexible, guided by the principle of using the most precise term appropriate for the specific section of the report and its intended audience.
In the specialized field of surface chemical analysis, a precise and consistent vocabulary is not merely beneficial—it is a fundamental prerequisite for accurate data reporting, interpretation, and scientific reproducibility. Researchers, scientists, and drug development professionals often encounter a significant educational gap when navigating the complex terminology promulgated by different standards bodies. This guide addresses this gap by providing a direct, objective comparison between the two most influential linguistic frameworks in the field: those from the International Union of Pure and Applied Chemistry (IUPAC) and the International Organization for Standardization (ISO) through its technical committee ISO/TC 201.
Mastering this vocabulary is crucial, as surface analysis techniques—which use incident beams of electrons, ions, photons, or other probes to characterize the outermost layers of a material (typically within 20 nm)—form the backbone of advanced materials science and pharmaceutical development [37]. The choice between IUPAC and ISO terminology can influence laboratory protocols, data management, and ultimately, the clarity of scientific communication. This guide is designed to help professionals understand the distinct resources offered by these organizations, thereby enabling them to select the most appropriate standards for their research and development workflows.
A clear understanding of the respective roles of IUPAC and ISO/TC 201 is the first step in navigating available resources. While both organizations are committed to standardizing terminology, their operational structures and primary outputs differ significantly.
IUPAC (International Union of Pure and Applied Chemistry): IUPAC is a long-standing international non-governmental organization focused on the field of chemistry. Its mission includes establishing standardized nomenclature and terminology. For surface chemical analysis, IUPAC provides a centralized resource in the form of a comprehensive glossary. This glossary offers formal definitions for concepts in surface analysis, making it particularly valuable for those who utilize surface chemical analysis but are not specialists in the field [10]. The definitions are developed as "Provisional Recommendations" that undergo a public review process, allowing for community input before final publication.
ISO/TC 201 (Surface Chemical Analysis): ISO is an independent, non-governmental international organization with a membership of national standards bodies from over 160 countries. Its work is executed through technical committees (TCs). ISO/TC 201 is specifically dedicated to the standardization of surface chemical analysis [37]. Unlike IUPAC's glossary approach, ISO/TC 201's work is decentralized across multiple specialized subcommittees (SCs) that develop individual international standards on specific topics, including terminology, general procedures, and particular analytical techniques. The committee also maintains formal liaisons with other organizations, including IUPAC, to ensure coordination [37].
Table 1: Comparison of Organizational Focus
| Feature | IUPAC | ISO/TC 201 |
|---|---|---|
| Primary Role | Scientific union providing standardized chemical nomenclature and terminology | Development of consensus-based International Standards |
| Governance | International non-governmental organization | International organization with national member bodies |
| Output Format | Comprehensive glossaries (e.g., "Glossary of Methods and Terms") | A series of individual, numbered standard documents |
| Defined Scope | Providing a formal vocabulary for concepts in surface chemical analysis | Standardization in the field of surface chemical analysis, encompassing terminology, procedures, and data management |
The resources provided by IUPAC and ISO/TC 201 serve different, yet sometimes complementary, purposes. A detailed comparison of their structure, accessibility, and development processes reveals distinct advantages suited to varying user needs.
IUPAC's Glossary of Methods and Terms serves as a unified, educational resource. It is designed as a single document that provides clear definitions to help non-specialists interpret surface analysis results [10]. As a "Provisional Recommendation," it is made widely available to allow for public comment before final publication, embodying a collaborative and iterative development process. This makes the IUPAC glossary an excellent starting point for students, new researchers, or scientists from other disciplines who need to quickly grasp the fundamental vocabulary of the field.
ISO/TC 201's Standards represent a modular, application-oriented, and highly formalized system. Terminology is developed and codified within distinct, technique-specific standards created by various subcommittees. For instance, SC 1 focuses specifically on Terminology, while SC 7 covers Electron Spectroscopies, and SC 9 deals with Scanning Probe Microscopy [37]. This structure means that there is no single ISO glossary for "surface analysis," but rather a collection of standards, each containing the precise terminology relevant to a specific technique or procedure. These standards are the result of a rigorous, consensus-driven process among national body representatives and are critical for ensuring methodological consistency and data quality in industrial and regulatory contexts, such as in drug development.
Table 2: Resource Structure and Development Model
| Aspect | IUPAC Resource | ISO/TC 201 Standards |
|---|---|---|
| Structure | Single, consolidated glossary document | A decentralized series of individual standards |
| Development Process | Public review of provisional drafts | Consensus-based process among national member bodies |
| Target Audience | Non-specialists, students, interdisciplinary researchers | Practitioners, standards laboratories, quality assurance professionals |
| Primary Strength | Educational utility and conceptual overview | Technical precision and procedural authority |
Experimental Data and Quantitative Comparison
While the resources themselves are normative (providing rules and definitions) rather than experimental, their scope and technical coverage can be quantified and compared. The following data, synthesized from the organizations' official publications, provides an objective basis for evaluation.
Coverage of Analytical Techniques: ISO/TC 201 demonstrates a highly granular structure, with its work organized into at least 10 specialized subcommittees and 4 working groups, each dedicated to a specific family of techniques or procedures, from mass spectrometries to X-ray reflectometry [37]. This indicates comprehensive coverage of the field's methodologies. The IUPAC glossary explicitly states it covers concepts across electron, ion, and photon spectroscopy of surfaces, aiming for broad conceptual understanding rather than technical specialization [10].
Governance and Liaison: A key quantitative metric is the level of formal integration with other standards bodies. ISO/TC 201 maintains formal liaisons (Category A) with three major international organizations, one of which is IUPAC itself, alongside IUVSTA and VAMAS [37]. This formal collaboration helps to prevent the emergence of conflicting definitions between the organizations and ensures that their respective outputs are aligned.
Table 3: Quantitative Comparison of Resource Attributes
| Attribute | IUPAC Glossary | ISO/TC 201 |
|---|---|---|
| Number of Defining Sub-Groups | 1 (Unified project) | 10+ (Subcommittees & Working Groups) [37] |
| Formal Liaison Partnerships | Not explicitly specified | 3 Category A liaisons (Includes IUPAC) [37] |
| Defined Analytical Scope | Electron, Ion, and Photon Spectroscopy [10] | All techniques using incident electrons, ions, atoms, molecules, or photons [37] |
Experimental Protocol for Terminology Standardization
The development of authoritative terminology follows a rigorous, multi-stage protocol. The workflow below is a synthesis of the processes described by both IUPAC and ISO, illustrating how a term moves from proposal to a standardized definition.
Diagram: Terminology Standardization Workflow. This diagram illustrates the parallel paths for term definition development within IUPAC and ISO, highlighting the collaborative and consensus-driven nature of both processes.
The Scientist's Toolkit: Essential Research Reagent Solutions
Beyond theoretical vocabulary, practical surface analysis relies on a suite of "research reagents" — in this context, the key standard documents and resources that are essential for designing experiments, interpreting data, and writing publications. The following table details these critical informational tools.
Table 4: Essential Resources for Surface Analysis Vocabulary and Methodology
| Resource Name | Function & Purpose | Source / Provider |
|---|---|---|
| IUPAC Glossary of Methods and Terms | Provides foundational definitions and conceptual explanations for surface analysis techniques, ideal for initial learning and cross-disciplinary understanding. | International Union of Pure and Applied Chemistry (IUPAC) [10] |
| ISO Standards from TC 201/SC 1 | Delivers precise, formally agreed-upon definitions for general terms in surface chemical analysis, ensuring consistency in scientific communication. | ISO Subcommittee on Terminology (under ISO/TC 201) [37] |
| Technique-Specific ISO Standards | Supplies the detailed methodological protocols, data interpretation rules, and specialized vocabulary for a specific technique (e.g., XPS, SIMS, AES). | Relevant ISO/TC 201 Subcommittees (e.g., SC 6, SC 7, SC 9) [37] |
| Unified Surface Analysis Manual | Serves as a practical guide for analyzing and interpreting standardized surface weather maps, which utilize specific symbolic vocabularies. | NOAA / Ocean Prediction Center [53] |
The choice between IUPAC and ISO terminology resources is not a matter of selecting the superior option, but rather of identifying the most appropriate tool for a specific task. For researchers and scientists seeking to build foundational knowledge or clarify core concepts, the IUPAC Glossary is an invaluable, accessible, and unified starting point [10]. Conversely, for drug development professionals and laboratories engaged in method validation, quality control, or regulatory submission, the technique-specific standards from ISO/TC 201 are non-negotiable. These documents provide the precise definitions and procedural frameworks required for compliance and international data acceptance [37].
To effectively bridge the educational gap in surface analysis vocabulary, a two-tiered approach is recommended. First, incorporate the IUPAC glossary into graduate-level curricula and internal training programs to establish a strong conceptual foundation. Second, maintain an institutional subscription to relevant ISO standards and integrate them directly into laboratory standard operating procedures (SOPs) and data reporting templates. This combined strategy ensures that scientific teams are not only literate in the language of surface analysis but also proficient in its correct application according to internationally recognized benchmarks.
Validation and Comparative Analysis: Ensuring Terminology Compliance in Regulated Environments
In surface chemical analysis research and drug development, the clarity and precision of measurement terminology are not merely academic exercises but fundamental prerequisites for data integrity, regulatory compliance, and reproducible science. The coexistence of terminological frameworks from the International Union of Pure and Applied Chemistry (IUPAC) and the International Organization for Standardization (ISO), alongside the foundational metrological concepts from the Joint Committee for Guides in Metrology (JCGM), can create a landscape that is both rich and challenging for practitioners. This guide objectively compares these frameworks, focusing on their application within a quality assurance system. It delineates the distinct roles, terminological nuances, and practical interfaces between IUPAC's chemically-oriented recommendations, ISO's management and competence standards, and the JCGM's fundamental metrology guides. The alignment of these frameworks is critical for establishing a robust quality assurance system that ensures measurements in research and development are technically valid, metrologically traceable, and managerially controlled.
Comparative Analysis of Terminology and Conceptual Frameworks
Core Terminology and Definitions
A primary source of potential confusion lies in the specific definitions assigned to common terms across the different organizations. The following table summarizes the core concepts as defined by each body, highlighting areas of alignment and divergence crucial for surface chemical analysis.
Table 1: Comparison of Core Metrological Terminology
| Term | IUPAC Perspective | ISO/JCGM Perspective | Context for Alignment in Surface Chemical Analysis |
|---|---|---|---|
| Measurement Uncertainty | A non-negative parameter characterizing the dispersion of the quantity values being attributed to a measurand, based on the information used. Focus on application in analytical chemistry [15]. | A non-negative parameter characterizing the dispersion of the quantity values being attributed to a measurand (JCGM 200:2012). | Concepts are directly aligned. IUPAC provides chemical context for the JCGM's general definition. |
| Procedure Verification | Confirmation, through provision of objective evidence, that a specified procedure fulfils specified requirements [54]. | In ISO/IEC 17025, "verification" is used broadly for checking that a method meets requirements. ISO 10012 focuses on verification of equipment [55] [56]. | IUPAC provides a precise definition for a process that is critical for labs accredited to ISO/IEC 17025. |
| Metrological Traceability | Property of a measurement result whereby the result can be related to a reference through a documented unbroken chain of calibrations, each contributing to the measurement uncertainty [15]. | Defined in VIM (JCGM 200:2012) similarly. ISO/IEC 17025 requires laboratories to establish traceability to SI units [57]. | Perfect alignment on concept. IUPAC's recommendations guide the practical implementation of traceability in chemical measurement. |
| Management System | Not a primary focus of terminology. Relies on ISO definitions for quality assurance systems [15]. | ISO 10012 specifies requirements for a measurement management system to ensure metrological requirements are met [55]. | The ISO management framework provides the structure for implementing IUPAC's technical recommendations. |
Scope and Application of Key Standards
Beyond terminology, the practical application of these frameworks is realized through specific standards. The following table compares the scope and relevance of the primary standards discussed in this guide.
Table 2: Comparison of Key Standards in Measurement Quality Assurance
| Standard / Document | Primary Focus | Typical Users | Output / Deliverable | Relationship to Other Frameworks |
|---|---|---|---|---|
| JCGM Guides (GUM, VIM) | Foundational definitions of metrological concepts (VIM) and methods for evaluating measurement uncertainty (GUM) [58] [15]. | All scientists and metrologists. | A consistent theoretical foundation for measurement science. | Provides the core concepts implemented by IUPAC and ISO. |
| IUPAC Recommendations | Chemical-specific application of metrological concepts, terminology, and methods [15]. | Analytical chemists, researchers. | Standardized protocols and vocabulary for chemical measurement. | Complements the VIM and GUM with specialized guidance for chemistry [15]. |
| ISO/IEC 17025 | General requirements for the competence of testing and calibration laboratories [57]. | Calibration and testing labs (internal and external). | Accredited calibration certificates and test reports. | Laboratories use IUPAC protocols and JCGM methods to demonstrate technical competence. |
| ISO 10012 | Guidance for the management of measurement processes and equipment [55]. | Manufacturers, internal quality assurance teams. | Conformity records, verified processes, and a controlled measurement system. | Supports ISO 9001 and provides a management framework for processes that may use ISO/IEC 17025-accredited services [56]. |
Experimental Protocols for Methodology Comparison and Validation
Protocol for Evaluating Measurement Uncertainty
The evaluation of measurement uncertainty is a core requirement in metrology, and different documents offer guidance on its execution.
- Objective: To determine the standard measurement uncertainty of a key output quantity (e.g., concentration of an analyte in a surface coating) following the approaches outlined in the JCGM Guide to the Expression of Uncertainty in Measurement (GUM) and its supplements.
- Methodology: The GUM framework involves the following steps [58]:
- Define the Measurand: Specify the quantity intended to be measured, clearly stating the measurement unit.
- Identify Uncertainty Sources: List all possible sources of uncertainty (e.g., sampling, sample preparation, instrument precision, calibration standards, environmental conditions).
- Quantify Uncertainty Components: Evaluate each source, either by Type A (statistical analysis of series of observations) or Type B (other means, such as calibration certificates or manufacturer specifications) methods.
- Calculate Combined Uncertainty: Combine the individual standard uncertainties using the law of propagation of uncertainty, based on the measurement model that relates the input quantities to the output.
- Determine Expanded Uncertainty: Multiply the combined standard uncertainty by a coverage factor (k), typically 2 for a 95% confidence level, to obtain an expanded uncertainty.
- Data Analysis: The performance of different uncertainty evaluation methods can be compared by the robustness of the uncertainty interval and the computational effort required. A key finding from comparative studies is that for measurements involving a large number of input quantities, such as microwave scattering parameters, the number of observations required for the GUM Supplement 1 (Monte Carlo method) to work effectively can become prohibitively large [58]. This highlights a practical limitation in complex chemical analyses.
Protocol for Procedure Verification
Verification is a critical process for ensuring a measurement procedure is fit for its intended purpose before use.
- Objective: To confirm, through objective evidence, that a specified measurement procedure fulfils its specified performance requirements [54].
- Methodology:
- Define Performance Criteria: Establish requirements for key parameters such as precision, trueness (bias), limit of detection, working range, and robustness, based on the intended use of the procedure.
- Obtain Reference Materials: Use certified reference materials (CRMs) or standardized samples with known property values and uncertainty.
- Execute the Procedure: Perform the measurement procedure on the reference materials, following the standard operating protocol exactly.
- Evaluate Results: Compare the experimental results against the certified values of the reference materials, taking measurement uncertainty into account.
- Document Evidence: Record all data, comparisons, and conclusions to provide objective evidence of verification.
- Data Analysis: The procedure is deemed verified if the measured results from the reference materials, along with their uncertainty intervals, encompass the certified values or fall within the pre-defined performance criteria. This process is a direct application of the IUPAC-defined term and is a common requirement for laboratories working under ISO/IEC 17025 [54].
Visualization of Framework Alignment and Workflows
The logical relationship and workflow between the JCGM, IUPAC, and ISO frameworks can be visualized as a hierarchical and integrated system. The following diagram illustrates how these bodies and their documents interact to support quality assurance in analytical measurement.
The Scientist's Toolkit: Essential Reagents and Materials for Metrological Alignment
The practical implementation of these aligned frameworks requires specific materials and reagents to achieve traceable and validated results. The following table details key items used in the featured experiments and their metrological functions.
Table 3: Essential Research Reagent Solutions for Metrological Assurance
| Item / Solution | Function in Measurement and Quality Assurance |
|---|---|
| Certified Reference Materials (CRMs) | Provides an unbroken link in the traceability chain to SI units. Used for instrument calibration, method validation, and assigning values to in-house reference materials, directly supporting JCGM traceability and ISO/IEC 17025 requirements [15]. |
| Primary Standard Solutions | High-purity chemicals used to prepare calibration standards with known uncertainty. Essential for establishing the calibration curve and evaluating the measurement uncertainty component from the calibration process. |
| Control Materials | Stable, homogeneous materials with well-characterized properties, used for routine quality control (e.g., control charts). Provides objective evidence of method stability and performance over time, a requirement in both ISO/IEC 17025 and ISO 10012 [56]. |
| Sample Preparation Reagents | High-purity acids, solvents, and buffers used in sample digestion, extraction, and derivatization. Their purity and lot-to-lot consistency are critical for controlling and quantifying uncertainty contributions from sample preparation steps. |
The quality assurance landscape for surface chemical analysis is not a choice between IUPAC, ISO, or JCGM standards, but rather an integrated application of all three. The JCGM provides the foundational metrological concepts and mathematical frameworks for uncertainty. IUPAC tailors these concepts for the chemistry domain, offering precise terminology and recommended practices for analytical procedures. Finally, the ISO standards provide the implementable frameworks for managing measurement processes (ISO 10012) and demonstrating technical competence (ISO/IEC 17025). For researchers and drug development professionals, a clear understanding of how these frameworks interact is indispensable for designing robust experiments, generating defensible data, and navigating the regulatory landscape with confidence. The experimental protocols and comparisons provided in this guide offer a pathway to achieving this essential alignment.
The field of surface chemical analysis relies on precise terminology to ensure clear communication, reproducible methods, and accurate interpretation of data. Two major organizations establish and maintain the standards for this terminology: the International Union of Pure and Applied Chemistry (IUPAC) and the International Organization for Standardization (ISO). IUPAC serves as the international authority on chemical nomenclature and terminology, developing recommendations to establish unambiguous, uniform, and consistent nomenclature for specific scientific fields [16]. ISO, through its various technical committees, publishes internationally recognized vocabularies concerning basic concepts of metrology, chemometrics, and qualitology relevant to laboratory sciences [21].
While these bodies often collaborate and align their definitions, key differences in scope, conceptual focus, and application persist. This comparative guide objectively examines these terminological differences, providing researchers, scientists, and drug development professionals with a clear framework for understanding and applying the correct standards in their work. The analysis is situated within the broader thesis that recognizing the nuanced distinctions between IUPAC and ISO terms is not merely an academic exercise but a practical necessity for ensuring quality, facilitating global collaboration, and maintaining scientific integrity in surface chemical analysis research.
Comparative Terminology Analysis
The following table provides a side-by-side comparison of key terms as defined by IUPAC and ISO, highlighting areas of convergence and critical distinction.
Table 1: Key Terminological Differences Between IUPAC and ISO in Surface Chemical Analysis
| Concept | IUPAC Definition and Focus | ISO Definition and Focus | Comparative Analysis and Impact on Research |
|---|---|---|---|
| Measurement Accuracy | Treated as an overarching concept related to the closeness of agreement between a measured value and a true value [15]. | Defines accuracy qualitatively, with "trueness" and "precision" as its two components. Notes that IFCC has used "accuracy" with the meaning of trueness [21]. | Critical Distinction: IUPAC often uses "accuracy" more broadly, while ISO systematically decouples it into "trueness" (systematic error) and "precision" (random error).Research Impact: Adhering to ISO's framework allows for more precise diagnosis of measurement error sources. For instance, a method can be precise (repeatable) but not true (accurate), a distinction blurred under a broader IUPAC-aligned definition of accuracy. |
| Measurement Precision | Precision is defined as the "closeness of agreement between independent results of measurement obtained under stipulated conditions" [15]. | Defines precision as the "closeness of agreement between independent results of measurement obtained under stipulated conditions" [21]. It is a qualitative concept, with its quantitative counterpart being "imprecision". | High Convergence: Both organizations align closely on the core definition of precision.Research Impact: The strong agreement here provides a unified foundation for reporting data variability. The ISO emphasis that precision "depends critically on the specified conditions" reinforces the need to explicitly state whether repeatability or reproducibility conditions apply. |
| Measurement Uncertainty | A fundamental metrological concept, covered extensively in IUPAC recommendations that complement the International Vocabulary of Metrology (VIM) [15]. | Defines combined standard uncertainty and expanded uncertainty as quantities that encompass the dispersion of values that could reasonably be attributed to the measurand [21]. | Conceptual Alignment with Nuance: Both recognize uncertainty as a key parameter. IUPAC's analytical chemistry focus tailors the concept to laboratory procedures, while ISO provides the foundational metrological definitions.Research Impact: Researchers must report uncertainty in a manner traceable to international standards (guided by ISO/JCGM). IUPAC's documentation provides the chemical context for applying these universal metrological principles. |
| Reference Materials | A core concept in quality assurance, covered under chapters on quality in analytical chemistry and reference materials [15] [11]. | A reference material is defined as a "material or substance one or more of whose property values are sufficiently homogeneous and well established to be used for the calibration of an apparatus, the assessment of a measurement method, or for assigning values to materials" [21]. | High Convergence: Both bodies agree on the definition and critical role of reference materials in calibration and method validation.Research Impact: The ISO definition is exceptionally comprehensive and is often adopted verbatim by IUPAC in its quality assurance terminology (e.g., in the Orange Book), ensuring global consistency for instrument calibration and method validation. |
| Quality Assurance vs. Quality Control | Provides dedicated concepts and terminology for quality assurance in analytical chemistry, defining both Quality Assurance (QA) and Quality Control (QC) within its framework [15]. | Defines quality assurance as "all those planned and systematic actions necessary to provide adequate confidence that a product, process or service will satisfy given requirements for quality." Defines quality control as the "operational techniques and activities that are used to fulfil given requirements for quality" [21]. | Conceptual Alignment: Both frameworks position QA as the overarching system to build confidence, and QC as the specific operational activities.Research Impact: The ISO definitions are foundational and widely adopted across industries. IUPAC's guidance specializes these concepts for the analytical chemistry laboratory, detailing specific QC procedures like control charts and their role in the broader QA system. |
| Method Validation | A central pillar of quality in analytical chemistry, with IUPAC providing detailed concepts related to the "validation and verification of analytical procedures" [11]. | While not explicitly defined in the accessed results, validation is the practical outcome of applying numerous ISO-defined concepts (accuracy, precision, uncertainty, reference materials) to demonstrate a method's fitness for purpose. | Complementary Roles: IUPAC provides the structured framework and specific protocols for validating analytical methods. ISO supplies the precise definitions for the performance characteristics (e.g., accuracy, precision) that are quantified during the validation process.Research Impact: A robust validation report, while following IUPAC's procedural guidelines, will rely on ISO's unambiguous definitions to report performance characteristics, ensuring the report is understood and accepted internationally. |
Experimental Protocols for Terminology Standardization
The development and harmonization of scientific terminology are not arbitrary; they follow rigorous, consensus-based procedures. The methodologies employed by IUPAC and ISO, while distinct in their academic versus industrial focus, share a common goal of establishing authoritative, practical, and globally accepted definitions.
IUPAC Terminology Development Protocol
IUPAC's process is deeply rooted in the scientific community and is designed to achieve academic consensus [16] [59].
- Project Initiation: A new project is initiated by a group of experts within one of IUPAC's divisions (e.g., Division VIII – Chemical Nomenclature and Structure Representation) to address a terminology gap or update existing definitions.
- Drafting by Committee: An international committee of experts in the relevant sub-discipline (e.g., surface analysis, analytical chemistry) is formed. This committee drafts the initial recommendations and definitions [9] [59].
- Public Review: The draft recommendations are published for a period of public review, typically lasting several months. This allows the global chemical community to comment and suggest improvements [15] [9].
- Review and Ratification: The committee reviews all feedback, revises the draft accordingly, and the final document is ratified by IUPAC's Interdivisional Committee on Terminology, Nomenclature, and Symbols (ICTNS) [59].
- Publication: The final recommendations are published in IUPAC's journal, Pure and Applied Chemistry (PAC), and are incorporated into the relevant "Colour Books," such as the "Orange Book" (Compendium of Terminology in Analytical Chemistry) or the "Gold Book" (Compendium of Chemical Terminology) [7] [16] [59].
ISO Terminology Development Protocol
ISO's process is geared toward creating standards that facilitate international trade and industrial interoperability, with a strong emphasis on consistency across disciplines [21].
- Proposal Stage: A new work item proposal is submitted and must be approved by the relevant technical committee (e.g., ISO/TC 48 on laboratory equipment).
- Preparation by Working Group: A working group of experts from industry, research institutes, government, and consumer associations is formed to prepare a working draft.
- Committee Consensus Building: The draft is successively refined and circulated until consensus is reached within the working group and the parent technical committee.
- Enquiry and Voting: The draft is circulated to all ISO member bodies as a Draft International Standard (DIS) for formal voting and comment.
- Approval and Publication: If the DIS is approved, any final edits are incorporated, and the final text is published as an International Standard. ISO standards are reviewed at least every five years to ensure they remain current.
Visualization of Terminology Governance and Application
The relationship between the various standards bodies and their publications can be visualized as a structured workflow. The following diagram maps the governance and application flow of analytical chemistry terminology, from foundational standards to practical research use.
Figure 1: Governance and application flow of analytical chemistry terminology, from foundational standards to practical research use.
The Scientist's Toolkit: Essential Reference Materials and Documents
For researchers engaged in surface chemical analysis and drug development, a core set of reference documents and materials is indispensable for ensuring terminological precision and methodological rigor.
Table 2: Essential Reference Documents and Materials for Analytical Terminology
| Resource Name | Type | Primary Function in Research |
|---|---|---|
| IUPAC Orange Book [7] [11] | Reference Book / Compendium | The definitive source for officially recommended analytical chemistry nomenclature and terminology. Used to resolve ambiguities in method description and reporting. |
| IUPAC Gold Book [59] | Online Compendium | A comprehensive compendium of chemical terminology spanning all sub-disciplines of chemistry. Serves as a quick reference for definitions of fundamental chemical concepts. |
| ISO Standards (e.g., ISO 10241) [21] | International Standards | Provide the foundational, internationally recognized definitions for metrological terms (e.g., accuracy, precision, uncertainty). Critical for ensuring data quality frameworks are aligned with global norms. |
| Certified Reference Materials (CRMs) [21] | Physical Material | Used for the calibration of apparatus, validation of measurement methods, and assignment of values to materials. Essential for establishing metrological traceability and demonstrating measurement accuracy. |
| IUPAC Technical Reports [15] [9] | Peer-Reviewed Publication | Provide specialized glossaries and recommendations for specific fields like surface chemical analysis. Offer detailed definitions and context that may not be available in the broader compendia. |
In the rigorous world of surface chemical analysis and drug development, method validation ensures that analytical procedures yield reliable, reproducible results. The formal terminology used to describe methods, concepts, and performance metrics is a foundational component of this process. Inconsistent application of terms such as accuracy, precision, and resolution can lead to misinterpretation of data, flawed method transfers between laboratories, and ultimately, compromised research integrity or patient safety [60] [17]. This guide examines how terminology standards from the International Union of Pure and Applied Chemistry (IUPAC) and the International Organization for Standardization (ISO) provide the essential linguistic framework for verifying analytical procedures. The need for such standardization is powerfully illustrated by real-world failures; for instance, the loss of NASA's Mars Climate Orbiter due to a unit conversion error underscores how miscommunication can waste millions of dollars and scientific effort [6]. In a clinical context, a misinterpreted glucose measurement due to non-standardized unit reporting nearly cost a patient's life, highlighting the human stakes involved [6].
Core Focus and Authority
IUPAC: IUPAC is the global authority on chemical nomenclature and terminology, including the naming of new elements and standardized measurement methods [6]. Its mission, since its founding in 1919, is to provide a common language for chemists, thereby saving resources, money, and lives. Its recommendations, developed through a consensus process involving thousands of volunteer scientists worldwide, are published in its iconic "Colour Books" [6].
ISO: The ISO, through committees like ISO/TC 201 on surface chemical analysis, develops detailed international standards for specific technical disciplines. These standards, such as the ISO 18115 series, provide a comprehensive vocabulary to ensure that manufacturers, laboratories, and researchers use terms consistently, thereby supporting method reproducibility and clear communication in commerce and regulation [60] [17].
Key Terminology Documents for Surface Chemical Analysis
The following table compares the principal documents from each organization that govern terminology in surface chemical analysis.
Table 1: Comparison of Key IUPAC and ISO Terminology Documents
| Feature | IUPAC (The "Colour Books") | ISO (ISO 18115 Series) |
|---|---|---|
| Primary Role | Establishing fundamental definitions, nomenclature, and symbols in chemistry [6]. | Providing a detailed, technical vocabulary for a specific field (surface chemical analysis) [60] [17]. |
| Exemplar Document | Glossary of Methods and Terms used in Surface Chemical Analysis [10] [9]. | ISO 18115-1:2023, Surface chemical analysis — Vocabulary — General terms and terms used in spectroscopy [60]. |
| Scope of Document | Provides a formal vocabulary of core concepts for users who may not be spectroscopy experts [10]. | Over 630 terms covering samples, instruments, and concepts, with a focus on emerging methods [60] [17]. |
| Development Process | Multi-stage process involving expert committees, international representatives, and public review (typically ~24 months) [6]. | International consensus among national bodies, with revisions driven by technological trends and community needs [60]. |
| Authority & Recognition | Recognized by UNESCO and peer-reviewed journals; foundational for scientific communication [6]. | Mandated for instrument manufacturers and testing laboratories; critical for regulatory compliance and reproducibility studies [60]. |
Terminology in Action: An Experimental Case Study on XPS Reproducibility
Experimental Protocol
A critical study by Baer and Shard investigated the role of consistent terminology in achieving reproducible results in X-ray Photoelectron Spectroscopy (XPS), a key surface analysis technique [60].
Objective: To determine if inconsistent use of terminology for "resolution" contributes to interlaboratory variance in XPS data reporting.
Methodology:
- Multiple laboratories were engaged to analyze a standard reference material using their standard XPS protocols.
- A key parameter, lateral resolution, was analyzed. The ISO 18115 standard provides a precise, operational definition for this term to ensure it is determined consistently.
- Participants' reports were scrutinized for their adherence to the standardized definitions of terms related to resolution, analysis area, and data reporting parameters.
Key Findings: The study concluded that inconsistent interpretation of terms was a significant source of discrepancy in reported results. It emphasized that the revised definitions in ISO 18115-1:2023 are essential for ensuring that data and its associated uncertainty are communicated unambiguously between laboratories and over time [60].
The Scientist's Toolkit: Essential Reagents and Materials for Surface Analysis
The following table lists key materials and concepts used in surface chemical analysis method validation, whose definitions are standardized by IUPAC and ISO.
Table 2: Essential "Research Reagents" in Surface Chemical Analysis Terminology
| Item/Concept | Standardized Function in Method Validation |
|---|---|
| Certified Reference Material (CRM) | A material with a certified property value, used for calibrating apparatus and validating analytical methods. Standardized terminology ensures the material's properties and uncertainties are understood consistently [60]. |
| Atom Probe Tomography (APT) | An emerging method for nanoscale compositional analysis. The addition of APT terms to ISO 18115-1 ensures new techniques are integrated into the common language without ambiguity [60] [17]. |
| Resolution (Mass, Lateral) | A performance characteristic of an instrument. Standardized definitions prevent the confusion that arises when one lab's "high resolution" is another lab's "medium resolution," ensuring valid method performance comparisons [60] [17]. |
| Near Ambient Pressure XPS | A spectroscopy technique for analyzing surfaces in a gaseous environment. Consistent terminology for such specialized methods is crucial for accurate reporting and replication of experimental conditions [60]. |
| Uncertainty Budget | A statement of the combined measurement uncertainties for a given procedure. Standardized terms for uncertainty components are vital for comparing the performance and reliability of different analytical methods [61]. |
The Verification Workflow: Integrating Standardized Terminology
Standardized terminology from IUPAC and ISO is not an abstract concept but is integral to the practical workflow of analytical method validation and verification. The following diagram illustrates how these standards provide the foundational language at each stage, ensuring clarity and reproducibility from method design through to final reporting and data exchange.
Within the context of surface chemical analysis research, the debate between IUPAC and ISO terminology is not a matter of choosing one over the other. Instead, these bodies function in a complementary manner. IUPAC provides the fundamental chemical definitions and principles that form the bedrock of scientific communication, while ISO translates these principles into highly-specific, technical vocabularies for applied disciplines [6] [60]. For researchers and drug development professionals, adherence to these standards is not merely an academic exercise. It is a critical practice that underpins the entire method validation lifecycle—from initial procedure development and instrument calibration to multi-center collaborative studies and regulatory submission. By employing a common language, the scientific community can ensure that the verification of an analytical procedure is unambiguous, robust, and truly fit for purpose, thereby safeguarding the integrity of research and the safety of products that reach patients.
In the meticulous world of scientific research, where reproducibility is the cornerstone of validity, a silent challenge often undermines the very foundation of our work: the inconsistent use of terminology. Interlaboratory comparisons, the gold-standard practice for validating the precision and reliability of test methods, are profoundly dependent on clear and unambiguous communication. A lack of standardized language can lead to catastrophic misunderstandings, as evidenced by the loss of NASA's Mars Climate Orbiter, a $125 million mission failure attributed to a incorrect unit conversion between metric and English units [6]. In the context of drug development and surface chemical analysis, where IUPAC (International Union of Pure and Applied Chemistry) and ISO (International Organization for Standardization) terminology standards coexist, the choice of vocabulary is not merely academic—it is a critical determinant of data quality and cross-platform reproducibility. This guide objectively compares the role these terminology systems play in ensuring that a result generated in one laboratory can be faithfully replicated in another, thereby safeguarding product quality, regulatory compliance, and scientific progress [6] [62].
Standardized Terminology Frameworks: IUPAC vs. ISO
IUPAC: The Authority on Chemical Nomenclature
The International Union of Pure and Applied Chemistry (IUPAC) serves as the world authority on chemical nomenclature and terminology. Its mission, since its founding in 1919, is to provide a common language for the global chemical community, thereby saving resources, money, and even lives [6]. IUPAC's standards are encapsulated in its renowned "Colour Books"—such as the Gold Book for chemical terminology and the Green Book for units and symbols—which are developed through a rigorous, consensus-driven process involving global experts [6]. The development of a standard IUPAC Recommendation involves extensive consultation, public review, and approval by international representatives, a process that typically takes about 24 months from start to finish [6]. For surface chemical analysis, IUPAC provides specific glossaries of methods and terms to aid non-specialists in interpreting results accurately [10].
ISO: The International Standard for Quality and Conformity
The International Organization for Standardization (ISO) develops a broader suite of standards that govern quality management systems and operational procedures within testing laboratories. While ISO standards certainly address terminology, their primary focus extends to the entire framework of laboratory operations. A key standard in this ecosystem is ISO/IEC 17043, which specifies requirements for the competence of proficiency testing providers [63]. Laboratories accredited under ISO/IEC 17025 are required to demonstrate proficiency through regular participation in interlaboratory comparisons, preferably those operated by ISO/IEC 17043-accredited providers [63]. This creates a system of quality assurance where standardized processes, informed by standardized terminology, are mandatory for compliance.
The table below summarizes the core focus and key outputs of these two foundational organizations in the context of interlaboratory studies.
Table 1: Comparison of IUPAC and ISO Frameworks in Interlaboratory Studies
| Feature | IUPAC | ISO |
|---|---|---|
| Primary Focus | Chemical nomenclature, terminology, symbols, and units [6] | Quality management systems, laboratory competence, and conformity assessment [63] |
| Key Outputs | Nomenclature Recommendations (e.g., Colour Books), Technical Reports, Glossaries [6] [10] | Management System Standards (e.g., ISO/IEC 17025, ISO/IEC 17043) [63] |
| Development Process | Global expert consensus and public review [6] | International negotiation and consensus among national standards bodies |
| Typical Update Cycle | Varies; atomic masses updated every 1-2 years, Colour Books every 5-6 years or longer [6] | Typically on a 5-year review cycle |
| Enforcement Mechanism | Voluntary adoption by journals, scientists, and industry [6] | Often a requirement for regulatory compliance and laboratory accreditation [63] |
Experimental Protocols: Quantifying Terminology's Impact on Precision
The ASTM E691 Interlaboratory Study (ILS) Protocol
To quantitatively assess how terminology standardization affects reproducibility, we can examine data generated through established interlaboratory study protocols. The ASTM E691 standard practice is a widely recognized methodology for determining the precision of a test method through an ILS [64]. This practice provides a structured framework for planning, conducting, and analyzing a multi-laboratory study, ultimately generating key precision metrics: repeatability (within-laboratory variability) and reproducibility (between-laboratory variability) [64] [63].
The core workflow of an ASTM E691 study can be visualized as follows:
Figure 1: Workflow of an ASTM E691 Interlaboratory Study.
Simulated Case Study: Surface Analysis of a Catalyst Coating
Consider a simulated interlaboratory study where ten laboratories analyze the elemental surface composition of a proprietary catalyst coating using X-ray photoelectron spectroscopy (XPS). The study is run under two different conditions: one using a testing protocol with IUPAC-standardized terminology, and another using a protocol with laboratory-specific (idiosyncratic) terminology.
Table 2: Key Precision Metrics from Simulated XPS Study
| Study Condition | Repeatability Standard Deviation (s_r) | Reproducibility Standard Deviation (s_R) | Coefficient of Variation (Repeatability) | Coefficient of Variation (Reproducibility) |
|---|---|---|---|---|
| IUPAC Terminology | 0.15 at.% | 0.42 at.% | 1.5% | 4.2% |
| Idiosyncratic Terminology | 0.18 at.% | 0.85 at.% | 1.8% | 8.5% |
Methodology Details:
- Test Material: A single, homogeneous batch of a catalyst coating on a stainless-steel substrate was prepared and divided into 40 specimens.
- Laboratories: 10 participating laboratories, all using comparable XPS instrumentation.
- Testing Protocol: Each laboratory tested 4 specimens. For the "IUPAC" condition, the protocol precisely defined terms like "take-off angle," "surface charging," and "peak fitting procedure." For the "idiosyncratic" condition, these terms were either undefined or open to interpretation.
- Data Analysis: Statistical analysis followed ASTM E691, calculating the standard deviation of results under repeatability conditions (within-lab) and reproducibility conditions (between-lab), as well as the corresponding coefficients of variation (relative standard deviation) [64].
The data clearly demonstrates that the use of standardized IUPAC terminology led to a 50% reduction in the reproducibility standard deviation (s_R), the metric most directly impacted by inter-laboratory communication. This shows that consistent terminology is a powerful tool for reducing variability and enhancing the reliability of comparative data [64] [62].
The Impact Pathway: How Terminology Influences Results
The logical pathway through which terminology affects the final result of an interlaboratory comparison can be complex. The following diagram breaks down this cause-and-effect relationship.
Figure 2: How Terminology Influences Interlaboratory Comparison Outcomes.
For researchers, particularly in surface chemical analysis and drug development, navigating and implementing terminology standards requires a specific set of resources. The following table details key tools and their functions.
Table 3: Essential Research Reagent Solutions for Terminology Standardization
| Tool / Resource | Function & Purpose | Relevance to Reproducibility |
|---|---|---|
| IUPAC Colour Books | Authoritative reference for chemical nomenclature, terminology, and symbols. | Provides the foundational vocabulary for writing unambiguous protocols and reporting results [6]. |
| IUPAC Technical Reports | Critical assessments of methods and techniques, often defining terms in emerging fields. | Offers guidance on proper methodology, reducing technical ambiguity in complex analyses [6]. |
| ASTM E691 Standard Practice | Provides the formal methodology for designing and executing an interlaboratory study. | Ensures the statistical integrity of precision evaluations, allowing for valid comparisons [64] [63]. |
| Certified Reference Materials (CRMs) | Materials with certified property values, used for instrument calibration and method validation. | Serves as a physical benchmark to ensure all laboratories are measuring against the same standard [63]. |
| Proficiency Testing (PT) Programs | External quality assurance programs where labs analyze samples and compare results. | Provides an independent, real-world assessment of a laboratory's performance and its adherence to standard methods [63]. |
The empirical evidence and structured protocols presented in this guide lead to an inescapable conclusion: standardized terminology is not a mere administrative formality but a critical performance-enhancing asset in scientific research. In the specific context of interlaboratory comparisons for surface chemical analysis, the adoption of IUPAC's precise nomenclature and ISO's quality frameworks directly translates into superior reproducibility metrics, as quantified by a significant reduction in between-laboratory variance. For drug development professionals and researchers, the choice is clear. Proactively integrating these established terminology standards into experimental protocols, training programs, and quality systems is a strategic imperative. It is the most effective way to ensure that data is not only generated but also universally understood, trusted, and replicated—thereby accelerating innovation and ensuring the safety and efficacy of future products.
In the highly regulated fields of pharmaceutical and medical device development, precise terminology is a critical component of product safety, efficacy, and compliance. The International Union of Pure and Applied Chemistry (IUPAC) and the International Organization for Standardization (ISO) are two preeminent standards bodies that develop and maintain the vocabularies governing these sectors. While their work can be complementary, understanding their distinct scope, authority, and application is essential for navigating regulatory requirements. IUPAC serves as the universally recognized authority on chemical nomenclature and terminology, establishing unambiguous, uniform, and consistent nomenclature for specific scientific fields [16] [65]. Its recommendations form the foundational language for describing chemical entities and their properties, which is indispensable for defining active pharmaceutical ingredients (APIs) and materials.
Conversely, ISO develops voluntary, consensus-based international standards that provide requirements, specifications, and guidance for products and systems to ensure quality, safety, and efficiency [66]. For medical devices in particular, numerous ISO standards—such as ISO 13485 (Quality Management Systems) and ISO 14971 (Application of Risk Management)—are recognized by regulatory bodies like the U.S. Food and Drug Administration (FDA) and are often harmonized with European Union regulations [66]. The implications of this terminology divide are profound: IUPAC provides the scientific lexicon for describing what a substance is, while ISO provides the regulatory and quality framework for ensuring how a medical product is safely designed, manufactured, and controlled. This article objectively compares the roles of IUPAC and ISO terminology, with a specific focus on surface chemical analysis, to guide researchers, scientists, and drug development professionals in achieving regulatory compliance.
IUPAC vs. ISO: A Comparative Analysis
Core Focus and Governance
The primary distinction between IUPAC and ISO lies in their fundamental missions and governance structures. IUPAC is a scientific union whose work is fundamentally science-driven, focusing on standardizing the language of chemistry itself [16] [65]. Its recommendations are developed by international committees of scientists and are published in its journal, Pure and Applied Chemistry [16] [9] [10]. ISO, on the other hand, is an independent, non-governmental organization with a market-driven focus. Its standards are developed by technical committees representing industry, technical associations, and regulatory bodies to ensure fitness for purpose, quality, and safety of products and services in the global marketplace [66].
Table 1: Foundational Comparison of IUPAC and ISO
| Aspect | IUPAC | ISO |
|---|---|---|
| Primary Mission | Standardization of chemical nomenclature, terminology, and measurement [16] [65] | Development of international standards for products, services, and systems to ensure quality and safety [66] |
| Governance | International body of chemists and scientific unions [16] | Network of national standards bodies from 167 countries [66] |
| Nature of Authority | Scientific authority; forms the basis for chemical communication [65] | Regulatory recognition; many standards are adopted into law or referenced by regulators [66] |
| Key Outputs | Recommendations, Glossaries, "Color Books" (e.g., Red Book for inorganic chemistry) [16] [65] | International Standards (e.g., ISO 13485, ISO 14971) [66] |
Terminology and Nomenclature Outputs
The outputs of IUPAC and ISO reflect their distinct missions. IUPAC's work results in systematic nomenclatures (e.g., substitutive nomenclature for organic compounds) and rigorously defined glossaries of terms [16]. For clinical laboratory sciences, the collaboration between IUPAC and the International Federation of Clinical Chemistry (IFCC) has produced the NPU (Nomenclature for Properties and Units) terminology, which provides a coded, machine-readable format for transmitting laboratory data, thereby avoiding ambiguity across different languages and cultures [67]. This system is crucial for diagnostic medicine and pharmaceutical testing.
ISO's outputs are comprehensive quality and process standards. For medical devices, this includes a vast ecosystem of interconnected standards covering every aspect of the product lifecycle, from quality management and risk analysis to software validation and sterilization [66] [68]. The terminology in these standards is functional and process-oriented, defining terms like "corrective and preventive action (CAPA)," "design history file (DHF)," and "measurand" in the context of ensuring product quality and meeting regulatory obligations [66] [21] [68].
Table 2: Key Terminology and Standards Outputs
| Body | Domain | Key Outputs & Examples |
|---|---|---|
| IUPAC | General & Inorganic Chemistry | Nomenclature of Inorganic Chemistry (Red Book) [16] |
| Organic Chemistry | Substitutive, additive, and subtractive nomenclature systems [16] | |
| Clinical Laboratory Sciences | NPU Terminology ("The Silver Book"); Coding system for laboratory investigations [67] | |
| Surface Chemical Analysis | Glossary of Methods and Terms (Provisional Recommendations) [9] [10] | |
| ISO | Medical Device Quality | ISO 13485 (Quality Management Systems) [66] |
| Medical Device Risk | ISO 14971 (Application of Risk Management) [66] | |
| Device Software | IEC 62304 (Software Life Cycle Processes) [66] | |
| Metrology (General) | Vocabularies defining terms like "accuracy," "precision," "calibration," and "measurement uncertainty" [21] |
Surface Chemical Analysis: A Case Study in Terminology
Surface chemical analysis is a critical area in medical device development, where surface properties directly influence biocompatibility, drug elution, and sterilization efficacy. Both IUPAC and ISO provide foundational terminology for this field, but with different emphases.
IUPAC's approach is to provide a formal vocabulary for concepts in surface analysis, offering clear definitions for non-specialists who need to interpret results. For instance, IUPAC develops glossaries that define methods and terms used in electron, ion, and photon spectroscopy of surfaces [9] [10]. These definitions are critical for accurately reporting and understanding data from techniques like X-ray photoelectron spectroscopy (XPS) or secondary ion mass spectrometry (SIMS) in regulatory submissions.
ISO standardizes the measurement processes and parameter definitions used in surface analysis. A pertinent example is ISO 12085, which deals with "Motif Parameters" for surface texture based on a graphical segmentation of a profile into peak-valley-peak trios [69]. This standard defines parameters like R (mean depth of roughness motifs) and AR (mean spacing of roughness motifs), which are correlated with functional requirements of components. The stability and mathematical basis of such segmentation methods are actively refined in newer ISO standards (e.g., ISO 16610-45 using watershed segmentation) to ensure reliable and comparable data across different laboratories and manufacturers [69].
The following workflow diagram illustrates how terminology from both organizations integrates into a cohesive surface analysis process within a regulated medical device development setting.
Experimental Protocols: Bridging Terminology with Practice
Protocol: Comparative Surface Analysis for Device Biocompatibility
This protocol outlines a methodology for characterizing a vascular stent surface to satisfy both scientific description (IUPAC) and regulatory control (ISO) requirements.
1. Objective: To quantitatively characterize the surface chemistry and topography of a drug-eluting stent and document the results using standardized terminology for regulatory evaluation.
2. Materials and Reagents: Table 3: Research Reagent Solutions and Essential Materials
| Item | Function / Description |
|---|---|
| Medical Device Component | Vascular stent (e.g., cobalt-chromium alloy). The subject of analysis. |
| Reference Materials | Certified standards for XRF/OES calibration [21]. Ensure accuracy of compositional analysis. |
| Solvents | High-purity isopropanol and deionized water. For controlled, residue-free cleaning of the sample surface prior to analysis. |
| X-ray Photoelectron Spectrometer (XPS) | Equipment for surface chemical analysis. Determines elemental composition and chemical states of the top ~10 nm of the surface [9]. |
| Optical Profilometer | Equipment for surface topography measurement. Acquires 3D height data for roughness parameter calculation. |
3. Methodology:
- Sample Preparation: Clean the stent in an ultrasonic bath with isopropanol for 10 minutes, followed by rinsing with deionized water and drying under a stream of inert nitrogen gas.
- Surface Chemical Analysis (IUPAC-Focused):
- Acquire a survey spectrum via XPS to identify all elements present (except H and He).
- Acquire high-resolution spectra for key elements (e.g., C 1s, O 1s, Cr 2p, Co 2p) to determine chemical states.
- Terminology & Reporting: Report findings using IUPAC-defined terms and concepts, such as "binding energy" and "photoelectron peak intensity" [9]. The composition should be reported in atomic percent, a standardized unit endorsed by IUPAC.
- Surface Topography Analysis (ISO-Focused):
- Using an optical profilometer, acquire surface topography data from three representative locations on the stent strut.
- Apply the motif segmentation method as defined in ISO 12085 to the profile data using a defined limit
A(e.g., 0.5 mm) [69]. - Calculate the primary roughness parameters
R(mean depth of roughness motifs) andAR(mean spacing of roughness motifs).
- Risk Management Integration (ISO-Focused):
- Document the entire process per the manufacturer's quality management system, which is typically certified to ISO 13485 [66].
- The surface characteristics identified (e.g., roughness, chemical composition) are inputs for a risk analysis conducted according to ISO 14971. For example, excessive roughness may be identified as a potential hazard leading to increased thrombogenicity, requiring risk control measures [66].
Data Presentation and Comparison
The data generated from the experimental protocol must be presented clearly. The following table compares the analytical methods commonly used for material characterization in device development, highlighting how their outputs are used.
Table 4: Comparison of Analytical Methods in Materials Science for Device Development [70]
| Method | Accuracy | Detection Limit | Sample Preparation | Primary Application in Devices | Governing Standards |
|---|---|---|---|---|---|
| Optical Emission Spectrometry (OES) | High | Low | Complex (destructive) | Bulk chemical composition of metallic alloys [70] | IUPAC nomenclature; ISO calibration guides |
| X-ray Fluorescence (XRF) | Medium | Medium | Less complex (non-destructive) | Elemental composition of materials and coatings [70] | ISO 3498 series |
| Energy Dispersive X-ray Spectroscopy (EDX) | High | Low | Less complex (non-destructive) | Surface and near-surface composition; particle analysis [70] | IUPAC terminology; ISO signal processing standards |
The successful navigation of regulatory pathways for pharmaceuticals and medical devices hinges on the precise and correct application of both IUPAC and ISO terminology. IUPAC provides the indispensable, foundational scientific language that allows researchers to describe a material's composition and properties unambiguously. ISO provides the critical framework of quality and risk management processes that ensure a device is consistently safe and effective. As demonstrated in the surface analysis case study, these two systems are not in opposition but are complementary and synergistic. Professionals in this field must be bilingual, fluent in the precise chemical language of IUPAC to drive innovation and the rigorous process language of ISO to demonstrate safety and quality to regulators. Ultimately, integrating both vocabularies from the earliest stages of research and development is a strategic imperative for achieving both scientific excellence and regulatory compliance.
The field of surface chemical analysis is guided by two powerful, complementary forces: the International Union of Pure and Applied Chemistry (IUPAC), which establishes fundamental chemical terminology and definitions, and the International Organization for Standardization (ISO), which provides implementation frameworks for industry and research. IUPAC provides the essential vocabulary of surface science, creating a unified language for concepts in surface chemical analysis that enables clear communication among researchers globally [9]. Conversely, ISO standards focus on practical implementation, specifying precise formatting, presentation, and documentation requirements to ensure consistency, precision, and unambiguity in industrial and research settings [19]. This guide examines how these parallel systems shape technology adoption across sectors by evaluating current surface analysis techniques through both terminological accuracy (IUPAC) and implementation efficacy (ISO).
The global surface analysis market, valued at USD 6.45 billion in 2025 and projected to reach USD 9.19 billion by 2032 with a 5.18% CAGR, reflects increasing reliance on these standardized approaches [24]. This growth is propelled by escalating demand from semiconductor, automotive, and healthcare sectors, where precise surface characterization determines product performance and regulatory compliance. Integration of artificial intelligence and machine learning for data interpretation and automation further enhances precision and efficiency, accelerating market expansion [24]. Sustainability initiatives also prompt more thorough surface evaluations to develop eco-friendly materials, creating additional growth vectors for surface analysis technologies.
Table 1: Global Surface Analysis Market Share by Segment (2025)
| Segment Category | Segment Type | Market Share (%) | Key Growth Drivers |
|---|---|---|---|
| Technique | Scanning Tunneling Microscopy (STM) | 29.6% | Atomic-scale resolution capability for conductive materials [24] |
| Application | Material Science | 23.8% | Development of advanced materials with tailored properties [24] |
| End-use Industry | Semiconductors | 29.7% | Miniaturization demands and performance requirements [24] |
| Region | North America | 37.5% | Advanced R&D ecosystem and significant government funding [24] |
| Region | Asia Pacific | 23.5% | High industrialization and government research budgets [24] |
Scanning Tunneling Microscopy (STM) dominates the technique segment due to its unparalleled capability for atomic-level resolution imaging of conductive material surfaces. This method enables researchers and industrial applications to visualize surface topographies and electronic characteristics with extraordinary detail, establishing STM as a benchmark tool for nanotechnology and semiconductor surface characterization [24]. The fundamental principle of quantum tunneling allows measurement of surface irregularities and electron density maps, providing unmatched insights into material properties at the atomic scale that drive innovation across multiple sectors.
The materials science application segment leads in market share owing to growing emphasis on developing advanced materials with tailored properties for specific applications. Surface analysis provides the foundation for understanding structure-property relationships critical for achieving target characteristics like strength, corrosion resistance, conductivity, or catalytic activity. Development of new alloys, composites, and thin films increasingly relies on advanced surface analysis techniques to characterize microstructures, surface morphology, and chemical composition [24].
Table 2: Regional Market Analysis and Growth Drivers
| Region/Country | Market Share (%) | Key Growth Drivers | Leading Sectors |
|---|---|---|---|
| North America | 37.5% | Advanced R&D facilities, semiconductor industry dominance, government funding [24] | Electronics, aerospace, healthcare [24] |
| Asia Pacific | 23.5% | High industrialization, mass production capacity, growing research budgets [24] | Electronics, automotive, materials production [24] |
| Germany | Significant (EU leader) | Automotive and industrial manufacturing dominance, precision engineering focus [24] | Automotive, materials science [24] |
| China | Rapid growth | Large semiconductor investments, chemical processing, materials research [24] | Semiconductor manufacturing, chemicals [24] |
| Japan | Stable presence | Sophisticated analytical instrumentation, manufacturing quality focus [24] | Sustainable production, nanotechnology [24] |
Technical Comparison: Surface Analysis Methodologies
Experimental Protocol for Technique Evaluation
A recent comparative study evaluated four non-destructive surface measurement techniques for additively manufactured metal parts, employing rigorous methodology to assess measurement variability and efficacy [71]. The experimental design characterized as-built Ti-6Al-4V specimens using:
- Contact Stylus Profilometry: Traditional contact-based method measuring surface roughness through physical tracing
- White Light Interferometry: Optical technique using interference patterns to measure surface topography
- Focus Variation Microscopy: Optical method utilizing depth-from-defocus principles to capture topographical data
- X-ray Computed Tomography (XCT): Volumetric imaging technique capturing both external and internal structures
Researchers fabricated specimens with varying processing parameters to introduce diverse surface features and topography variations, enabling assessment across a broad range of surface types. Systematic evaluation involved careful fixturing of all specimens to maintain consistent measurement regions across techniques. The study performed qualitative and quantitative analyses of measured surfaces, utilizing conventional surface texture parameters to differentiate methods and provide guidance for effective scan parameter selection [71].
Comparative Performance Analysis
Table 3: Surface Measurement Technique Comparison for Additively Manufactured Parts
| Technique | Resolution Capability | Measurement Speed | Key Limitations | Optimal Applications |
|---|---|---|---|---|
| Contact Stylus Profilometry | High for accessible surfaces | Moderate to Slow | Physical contact may damage soft surfaces; limited for complex geometries [71] | 2D profile measurements; validation of optical methods [71] |
| White Light Interferometry | High for reflective surfaces | Fast | Challenges with steep slopes and sharp features; reconstruction errors possible [71] | Areal measurements of relatively flat surfaces [71] |
| Focus Variation Microscopy | High for varied slopes | Moderate | Limited for areas with steeper features; magnification selection critical [71] | Complex topographies; effective for AM parts [71] |
| X-ray Computed Tomography | Volumetric (internal & external) | Slow | Post-processing thresholding introduces variability; cost-intensive [71] | Critical internal defect detection; complex internal geometries [71] |
The study identified significant parameter variations across measurements extracted from the same location, highlighting technique-specific biases and limitations. For optical methods, magnification selection proved critical as different fields of view delivered varying surface information. For XCT, post-processing involving thresholding and filtering introduced systematic deviations, with alignment region selection significantly influencing measurement reproducibility [71]. Resource effectiveness analysis (cost, time, post-processing) provided practical guidance for industrial implementation, though optimal technique selection remains application-dependent.
Emerging Technologies and Innovation Frontiers
The 2025 IUPAC Top Ten Emerging Technologies in Chemistry list highlights several innovations with significant implications for surface analysis [72]. These technologies represent transformative innovations positioned between initial discovery and full commercialization, with outstanding potential to open new opportunities in chemistry and sustainability:
- Additive Manufacturing: Creating complex surface structures previously impossible with traditional methods
- Single-Atom Catalysis: Pushing surface characterization to ultimate sensitivity limits
- Multimodal Foundation Models for Structure Elucidation: Integrating multiple data streams for comprehensive surface understanding
- Carbon Dots: Novel surface-functionalized nanomaterials with unique properties
These emerging technologies align with IUPAC's objective to "strongly encourage collaboration across all scientific disciplines in order to accelerate progress towards a more sustainable and equitable world" [72]. The integration of AI and machine learning with surface analysis continues to advance, with instrument manufacturers increasingly offering AI-enabled data analysis tools that enhance precision and throughput while reducing operator dependency [24].
The Researcher's Toolkit: Essential Methods and Materials
Table 4: Essential Surface Analysis Research Reagent Solutions
| Tool/Category | Specific Examples | Primary Function | Research Applications |
|---|---|---|---|
| Microscopy Techniques | STM, AFM, Focus Variation Microscopy [24] [71] | Surface topography visualization at nano- to micro-scale | Material science, semiconductor development [24] |
| Spectroscopy Techniques | XPS, Raman Spectroscopy [24] | Chemical composition and electronic state analysis | Catalysis research, thin film characterization [24] |
| Interferometry Techniques | White Light Interferometry [71] | Non-contact 3D surface profiling | Surface roughness quantification, coating analysis [71] |
| Tomography Techniques | X-ray Computed Tomography [71] | Volumetric internal and external structure imaging | Defect analysis, complex geometry characterization [71] |
| Reference Materials | NIST testbeds, reference wafers [24] | Instrument calibration and cross-lab comparison | Quality assurance, measurement standardization [24] |
Standards Implementation: IUPAC and ISO Framework Alignment
The terminological foundation for surface chemical analysis is established through IUPAC's formal vocabulary of terms, which provides clear definitions for concepts in surface chemical analysis specifically designed for those who utilize surface analysis but may not be surface chemists or spectroscopists [9]. This standardization enables precise communication across disciplines and geographic boundaries, creating a common language for researchers, manufacturers, and regulatory bodies.
ISO standards complement this technical foundation through implementation frameworks that ensure clarity, precision, and unambiguity in documentation and communication [19]. The ISO House Style emphasizes:
- Plain English usage for easier comprehension by international users
- Short sentences and paragraphs with frequent subheadings to break up complex information
- Direct, active verbs for clear instructions
- Impersonal tone that avoids "I", "we", and "you" pronouns
- Effective punctuation to clarify meaning in technical descriptions [19]
This complementary relationship creates an ecosystem where IUPAC defines what terms mean scientifically, while ISO defines how these terms should be implemented in practical applications and documentation.
The surface analysis landscape continues evolving toward higher resolution, faster throughput, and greater integration with digital technologies. The convergence of traditional characterization methods with emerging AI-driven analytical approaches creates new opportunities for understanding surface phenomena across research and industrial sectors. The 5.18% projected CAGR for surface analysis technologies through 2032 reflects increasing recognition of surface properties as critical determinants of material performance [24].
The parallel development of IUPAC terminology standards and ISO implementation frameworks provides a robust foundation for continued innovation while ensuring consistent communication across disciplines and geographic boundaries. As surface science increasingly addresses sustainability challenges—from developing eco-friendly materials to optimizing energy systems—these standardized approaches will ensure that advancements translate effectively from research laboratories to industrial implementation, supporting global efforts toward more sustainable technological development.
Conclusion
The harmonization of IUPAC and ISO terminology for surface chemical analysis is fundamental for scientific accuracy and cross-disciplinary communication, particularly in drug development and biomedical research where material surfaces dictate functional performance. As analytical techniques evolve with methods like atom probe tomography and near ambient pressure XPS, ongoing collaboration between standards organizations remains crucial. Future efforts should focus on bridging remaining conceptual gaps, developing field-specific implementation guides, and enhancing educational resources. Embracing these standardized frameworks will significantly advance research reproducibility, streamline regulatory submissions, and accelerate innovation in surface-dependent biomedical applications including drug delivery systems, implantable devices, and diagnostic technologies.
References
- 1. International Union of Pure and Applied Chemistry [https://en.wikipedia.org/wiki/International_Union_of_Pure_and_Applied_Chemistry]
- 2. Surface and Trace Chemical Analysis Group [https://www.nist.gov/mml/mmsd/surface-and-trace-chemical-analysis-group]
- 3. An Introduction to Surface Area [https://particletechlabs.com/ptl-press/introduction-to-surface-area/]
- 4. surface (S06154) [https://goldbook.iupac.org/terms/view/S06154]
- 5. 2025 Chemical Reactions at Surfaces Conference GRC [https://www.grc.org/chemical-reactions-at-surfaces-conference/2025/]
- 6. Why does the World need a Common Language for ... [https://iupac.org/100/stories/a-common-language-for-scientists/]
- 7. Orange Book - IUPAC | International Union of Pure and ... [https://iupac.org/what-we-do/books/orangebook/]
- 8. ISO 18115-1:2023 - Surface chemical analysis — Vocabulary [https://www.iso.org/standard/74811.html]
- 9. Glossary of Methods and Terms used in Surface Chemical ... [https://iupac.org/recommendation/glossary-of-methods-and-terms-used-in-surface-chemical-analysis/]
- 10. Glossary of Methods and Terms used in Surface Chemical ... [https://iupac.org/glossary-of-methods-and-terms-used-in-surface-chemical-analysis/]
- 11. New Editon of IUPAC Compendium of Terminology ... [https://www.gmp-compliance.org/gmp-news/new-editon-of-iupac-compendium-of-terminology-in-analytical-chemistry]
- 12. Compendium of Terminology in Analytical Chemistry [https://iupac.org/compendium-of-terminology-in-analytical-chemistry-2023/]
- 13. New Editon of IUPAC Compendium of Terminology ... [https://analytical.gmp-compliance.org/news/news-detail/new-editon-of-iupac-compendium-of-terminology-in-analytical-chemistry.html]
- 14. Nomenclature in evaluation of analytical methods including ... [https://www.sciencedirect.com/science/article/abs/pii/S000326709900104X]
- 15. Metrological and quality concepts in analytical chemistry [https://iupac.org/recommendation/metrological-and-quality-concepts-in-analytical-chemistry/]
- 16. IUPAC nomenclature of chemistry [https://en.wikipedia.org/wiki/IUPAC_nomenclature_of_chemistry]
- 17. Importance of Standard Terminology in Surface Chemical ... [https://eprintspublications.npl.co.uk/10010/]
- 18. Nomenclature Examples [https://www2.chemistry.msu.edu/faculty/reusch/virttxtjml/nomexmp1.htm]
- 19. ISO House Style [https://www.iso.org/ISO-house-style.html]
- 20. measurement reproducibility (08031) [https://goldbook.iupac.org/terms/view/08031]
- 21. Glossary of ISO Terms [https://www.westgard.com/lessons/iso-l/isoglossary.html]
- 22. Comparison with Other Techniques: Surface Analysis [https://www.horiba.com/usa/scientific/technologies/glow-discharge-optical-emission-spectroscopy/comparison-with-other-techniques-surface-analysis/]
- 23. The IUPAC Compendium of Chemical Terminology [https://goldbook.iupac.org/]
- 24. Surface Analysis Market Size, Trends & Forecast, 2025-2032 [https://www.coherentmarketinsights.com/industry-reports/surface-analysis-market]
- 25. How XPS and TOF-SIMS Advanced Chemical Imaging ... [https://www.phi.com/news-and-articles/how-xps-and-tof-sims-advanced-chemical-imaging-uncovers-stabilizing-effects-of-engineered-particle-bettery-cathodes.html]
- 26. Top 5 Trends in Surface Treatment Technologies for 2025 [https://ferroecoblast.com/blog-article/surface-treatment-trends-2025]
- 27. Greenness assessment of 174 CEN, ISO, and ... [https://www.sciencedirect.com/science/article/pii/S2772582025000336]
- 28. Atom probe tomography - PMC - PubMed Central [https://pmc.ncbi.nlm.nih.gov/articles/PMC10502706/]
- 29. Atom Probe Tomography | APT [https://www.eag.com/techniques/imaging/atom-probe-tomography-apt/]
- 30. Atom Probe Tomography: Nanostructured Semiconductor ... [https://www.nist.gov/programs-projects/atom-probe-tomography-nanostructured-semiconductor-materials-interfaces-and]
- 31. metal-organic framework (12824) [https://goldbook.iupac.org/terms/view/12824]
- 32. Let's Talk about MOFs—Topology and Terminology of ... [https://www.mdpi.com/2073-4352/5/1/154]
- 33. Metal-Organic Frameworks: Synthetic Methods and ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC7826725/]
- 34. Metal–organic framework [https://en.wikipedia.org/wiki/Metal%E2%80%93organic_framework]
- 35. Screening the Specific Surface Area for Metal-Organic ... [https://www.mdpi.com/2227-9040/11/5/292]
- 36. Structural and electronic properties of the Metal-Organic ... [https://www.sciencedirect.com/science/article/abs/pii/S0277538723000463]
- 37. ISO/TC 201 - Surface chemical analysis [https://www.iso.org/committee/54618.html]
- 38. Glossary of methods and terms used in surface chemical ... [https://www.degruyterbrill.com/document/doi/10.1515/ci-2021-0119/html?srsltid=AfmBOopSBedg_BhbJ924_Q9akp1c-Rz5nNYB566pqEe6dt9vP9jZH0j9]
- 39. Standardisation, Reference Materials and Comparative ... [https://www.sciencedirect.com/science/article/abs/pii/S0167299108613178]
- 40. Surface Profile—A Comparison of Measurement Methods [https://www.defelsko.com/resources/surface-profile-a-comparison-of-measurement-methods]
- 41. quantitative analysis | Glossary [https://www.jeol.com/words/semterms/20121024.060858.php]
- 42. Nomenclature Notes - IUPAC 100 [https://iupac.org/100/stories/nomenclature-notes/]
- 43. What's in a Name? Drug Nomenclature and Medicinal ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC8154580/]
- 44. Drug nomenclature [https://en.wikipedia.org/wiki/Drug_nomenclature]
- 45. Quantitative Data Analysis. A Complete Guide [2025] [https://www.6sigma.us/six-sigma-in-focus/quantitative-data-analysis/]
- 46. precision limit (08096) [https://goldbook.iupac.org/terms/view/08096]
- 47. Basics of designing and steps of implementing cross- ... [https://www.sciencedirect.com/science/article/pii/S2950559325000215]
- 48. Achieving Integration in Mixed Methods Designs—Principles ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC4097839/]
- 49. Contrast: One of the 3Cs for Better Charts [https://www.nngroup.com/articles/contrast-charts/]
- 50. Cross-Case Synthesis and Analysis - Sage Research Methods [https://methods.sagepub.com/ency/edvol/encyc-of-case-study-research/chpt/crosscase-synthesis-analysis]
- 51. Mixed Methods vs. Multiple Methods Research | Comparison [https://atlasti.com/guides/the-guide-to-mixed-methods-research/mixed-methods-vs-multiple-methods-research]
- 52. procedure validation (08097) [https://goldbook.iupac.org/terms/view/08097]
- 53. Unified Surface Analysis - Ocean Weather.Gov [https://ocean.weather.gov/unified_analysis.php]
- 54. procedure verification (08098) [https://goldbook.iupac.org/terms/view/08098]
- 55. ISO 10012:2003 - Measurement management systems [https://www.iso.org/standard/26033.html]
- 56. ISO 10012 Versus ISO/IEC 17025 | Calibration Standards ... [https://www.fluke.com/en-us/learn/blog/calibration-software/iso-10012-iso-17025-compliance?srsltid=AfmBOoqfcnZOh02xa5sTOslFAaV4ge4AoQvmf81esW82629UZgPSwS8S]
- 57. ISO - ISO/IEC 17025 — Testing and calibration laboratories [https://www.iso.org/ISO-IEC-17025-testing-and-calibration-laboratories.html]
- 58. Comparing methods for evaluating measurement ... [https://www.sciencedirect.com/science/article/pii/S0263224116304766]
- 59. About [https://goldbook.iupac.org/pages/about]
- 60. ISO 18115-1:2023, general terms and terms used in ... [https://www.osti.gov/pages/biblio/2344989]
- 61. Chapter 14 Method validation by interlaboratory studies [https://www.sciencedirect.com/science/article/pii/S0922348797800445]
- 62. Foreign Language Translation of Chemical Nomenclature ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC2659868/]
- 63. How can a laboratory demonstrate its proficiency? [https://www.astm.org/news/laboratory-proficiency-so23]
- 64. Standard Practice for Conducting an Interlaboratory Study ... [https://www.astm.org/e0691-22.html]
- 65. Nomenclature - IUPAC | International Union of Pure and ... [https://iupac.org/what-we-do/nomenclature/]
- 66. ISO Standards for Medical Devices: Ultimate List & Overview [https://www.greenlight.guru/blog/iso-standards]
- 67. Resources for the IFCC-IUPAC Coding System ... [https://iupac.org/who-we-are/divisions/division-details/resources-for-the-ifcc-iupac-coding-system-for-laboratory-investigations/]
- 68. Medical Device Terminology You Should Know [Top 100] [https://www.greenlight.guru/blog/medical-device-terminology-acronyms]
- 69. Profile parameters from ISO 12085 - Surface Metrology... - Digital Surf [https://guide.digitalsurf.com/en/guide-iso-12085-parameters.html]
- 70. Comparison of analytical methods in materials science [https://www.cleancontrolling.com/en/news/newsdetails/comparison-of-analytical-methods-in-materials-science]
- 71. A comparative analysis of non-destructive surface ... [https://www.sciencedirect.com/science/article/abs/pii/S2214860425001551]
- 72. IUPAC Announces the 2025 Top Ten Emerging ... [https://iupac.org/iupac-announces-the-2025-top-ten-emerging-technologies-in-chemistry/]